Extracellular Vesicle (EV) Purification Kit"EXORPTION"
*This kit is intended for sale to healthcare professionals and researchers as it is a research reagent.
Click here for product inquiries and sample requests for EXORPTION, a purification kit for Extracellular Vesicle (EV) containing exosomes.
What is Great about Sanyo Chemical's Extracellular Vesicle (EV) Purification Kit “EXORPTION” ?
1
Simple operation and purification of Extracellular Vesicle (EV) in less than 90 minutes
"EXORPTION" is a purification kit developed by Sanyo Chemical Industries that provides a simple operation to obtain high purity Extracellular Vesicle (EV) with a high recovery rate in only 90 minutes. This kit uses a spin column containing special hydrogel beads that absorb biological samples that can be purified using a standard small tabletop centrifuge, eliminating the need for dedicated equipment. Compared to the currently popular ultracentrifugal method, the purification time is about 1/20th (90 minutes) yielding highly pure Extracellular Vesicle (EV) with less than 1/100th of the amount of foreign substances (mixtures other than those to be measured) with nearly 10 times the recovery volume.
2
Achieving both higher recovery and purification compared to the Ultracentrifugal method (UC) and to competitor kits
The greatest advantage of this method is the amount of Extracellular Vesicle (EV) recovered per sample, which is more than 10 times higher than that of the "gold standard" ultracentrifugal method (see figure below).
This feature contributes to highly sensitive detection of Extracellular Vesicle (EV) contained in extremely small amounts in body fluids, but it is also useful in drug discovery to obtain large amounts of Extracellular Vesicle (EV) for therapeutic use. Unlike some affinity purification methods, the final purified product does not contain any extra chemical substances, and the lipid bilayer structure of the Extracellular Vesicle (EV) is not destroyed, which are major advantages in drug discovery.
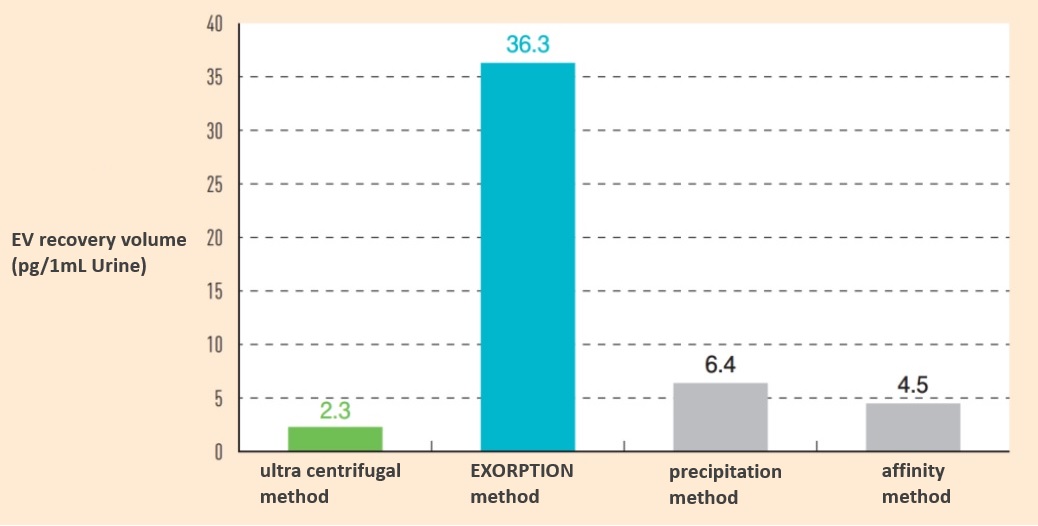
Fig. Extracellular Vesicle (EV) recovery (CD9/CD63 ELISA calculation)
Bioseparations were performed using urine from chronic kidney disease (CKD) patients by ultracentrifugation, EXORPTION, and commercial kits.
Ultracentrifugation was performed using the density gradient centrifugation method, and commercial kits were performed using protocols recommended by each company.
Extracellular Vesicle (EV) recovery is quantified by ELISA for both CD9/CD63 positivity (CD9/CD63 Exosome ELISA Kit, Human [Cat. No. EXH0102]).
What are Extracellular Vesicle (EV)?
Extracellular Vesicle (EV) are granule-like substances secreted by cells, whose surface contains lipids and proteins derived from the cell membrane, while the interior contains nucleic acids (microRNA, messenger RNA, DNA, etc.), proteins and other intracellular substances (see figure below). They are classified into three types according to their production pathway or size: exosomes, microvesicles, and apoptotic bodies. However, since these definitions and production pathways are very difficult and often confusing, the International Society for Extracellular Vesicle (ISEV), founded in 2011, has collectively named them "Extracellular Vesicle".
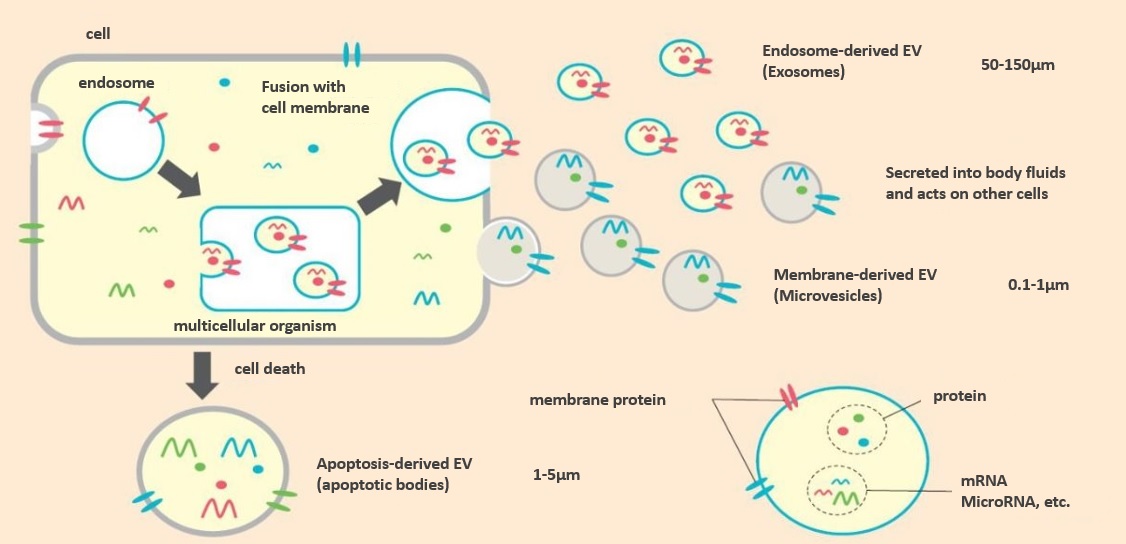
Fig. Extracellular Vesicle containing exosomes
Transmitters used for “cell-to-cell communication
Extracellular Vesicle (EV) secreted by cells circulate throughout the body in body fluids (blood, spinal fluid, saliva, urine) and have been shown to play a role in cell-to-cell communication by skillfully utilizing surface or internal molecules.
When another cell receives an Extracellular Vesicle (EV) circulating in the body, it undergoes various reactions and is involved in disease progression and therapeutic effects. For example, Extracellular Vesicle (EV) released by cancer cells have been shown to promote cancer metastasis, suppress immune cells, and induce neovascularization. Since Extracellular Vesicle (EV) contain information about the cells from which they are secreted, a new diagnostic method has been developed to detect diseases at a very early stage by collecting Extracellular Vesicle (EV) in body fluids and analyzing their disease information (see figure below).
In addition, Extracellular Vesicle (EV) released by some cells have been reported to have therapeutic effects such as improving renal function and suppressing inflammation, and their application is being considered for Alzheimer's disease, a neurodegenerative disease that is difficult to treat.
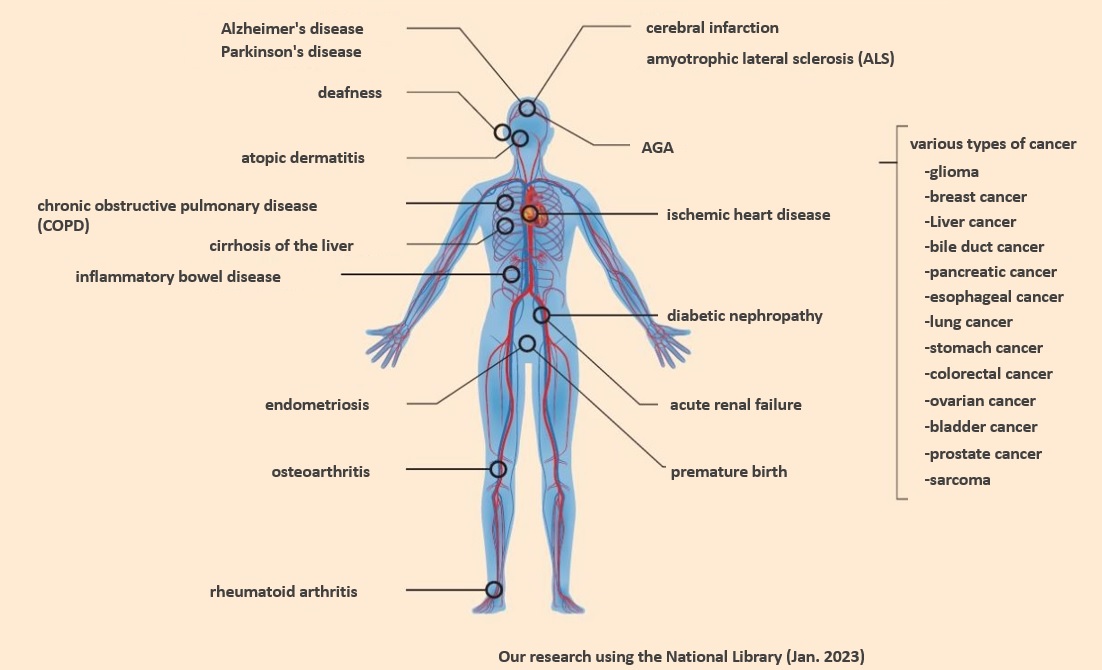
Fig. Examples of studies on treatment and diagnosis using Extracellular Vesicle (EV)
Challenges in the Development of Extracellular Vesicle (EV) Preparations
For industrial application, it is necessary to solve the following four issues, including the establishment of bioseparation technology.
| Technology Challenge | Selection of raw materials There are many issues such as the type of cells to be used as raw materials for Extracellular Vesicle (EV), quality control, culture methods, culture media, and handling cases that do not involve cultur Extracellular Vesicle (EV) purification method Purification methods are needed to solve the problems of processing large amounts of samples, contamination of impurities, size fractionation, and removal of Extracellular Vesicle (EV) that do not contain active ingredients. |
|---|---|
| Efficacy and Safety | Safety Need to establish quality control methods for safety of Extracellular Vesicle (EV) themselves, infectious substances and endotoxins, and prevention of contamination of culture medium components Regulation of active ingredients and dosage Clarification of active ingredients, mechanism of action, and amount of active ingredients, and establishment of an evaluation system for efficacy are required to ensure efficacy, as well as clarification of dosage units and measurement methods when administered into the body. |
Bioseparation of Extracellular Vesicle (EV)
Bioseparation is a technology for selectively separating cells, proteins, nucleic acids, and other biological substances from biological samples. It is an important technology that can be called the core of drug discovery, as it directly affects the sensitivity of tests for disease in diagnosis and the efficacy and side effects of drugs in treatment. Currently, the industry standard method of Extracellular Vesicle (EV) bioseparation is ultracentrifugation.
The general image of ultracentrifugation is that the target material accumulates at the bottom of the separator and can be collected, but obtaining Extracellular Vesicle (EV) in high purity using this method requires a very delicate technique in which the sample is placed in a liquid and the layer that appears at a specific height after centrifugation is sucked out and collected. This complex and time-consuming procedure with a recovery rate of less than 20% is a difficulty unique to Extracellular Vesicle (EV), and is the reason why bioseparation is said to be a barrier to the practical application of Extracellular Vesicle (EV). There is a great need for a solution to the above problem, and research reagents that enable bioseparation with a simpler operation other than the ultracentrifugation method are now on the market.
Comparison of bioseparation methods
A comparison of purification methods was conducted, including research reagents that can be bioseparated using a simpler procedure compared to the ultracentrifugal method (see table below).
| Mechanism | Processing time and man-hours | Performance Features | |
|---|---|---|---|
| Ultracentrifugal method | A method that uses centrifugal force to separate and collect Extracellular Vesicle (EV) into fixed fractions. Pellet down method and density gradient centrifugation are examples. | 1-3days | It has become the gold standard for Extracellular Vesicle (EV) research. Recovery efficiency and degree of purification are not high, and the influence of manual techniques is significant. Since there is no material other than the container to come in contact with, there is no need to consider the effects of reagents or other factors. |
| Filter method | Separation of foreign substances and Extracellular Vesicle (EV) using ultrafiltration filters, etc. | 30-120min | Operation is simple and there is little manual intervention. A combination of filters can be used to finely separate sizes, but the membranous Extracellular Vesicle (EV) are adsorbed and losses are high, and purification is difficult to achieve. |
| Precipitation method | A method of concentration separation by adding a polymer solution to a biological sample containing Extracellular Vesicle (EV) to precipitate the Extracellular Vesicle (EV). | 30-120min | The operation is simple and Extracellular Vesicle (EV) can be obtained in a short time. However, some foreign substances precipitate together with the Extracellular Vesicle (EV), making it difficult to achieve a high degree of purification. Also, the final purified product is contaminated with reagents. |
| Affinity method | Binding of Extracellular Vesicle (EV) to immobilized carriers to which antibodies or other substances have been bound, and then removing the binding and collecting the EV after washing away foreign substances. | a few hours | Generally, the degree of purification is high because of the use of affinity to separate from foreign substances. Scale-up is difficult because the immobilized carrier is manipulated for separation. Also, depending on the method used to bind Extracellular Vesicle (EV), the final purified product may contain reagents. |
| HAS method | A method for contacting a biological sample containing Extracellular Vesicle (EV) with hydrogel beads, supplementing the surface of the beads with Extracellular Vesicle (EV) and separating foreign substances (low molecular weight foreign substances inside the beads, high molecular weight foreign substances outside the beads), and collecting the Extracellular Vesicle (EV) on the surface | 70-90min | Highly purified Extracellular Vesicle (EV) can be obtained in a short time and in high yield. The operation is simple, so there is little manual intervention. |
Hydrogel Adsorption Separation (HAS) Method
The Hydrogel Adsorption Separation (HAS) method is an EV separation technique that utilizes Extracellular Vesicle (EV) adsorption on the surface of hydrogel.
EXORPTION - Extracellular Vesicle (EV) Purification Kit by HAS Method
-Time to complete specimen processing: approx. 1/10
-Recovery rate of Extracellular Vesicle (EV) per mL of specimen: approx. 10 times higher
-Amount of miscellaneous materials contained in specimens: approx. 1/100
(compared to density gradient ultracentrifugation)
HAS Method - Principle of Extracellular Vesicle (EV) Purification
In collaboration with a research group led by Professor Tatsuya Tominaga of the Department of Biofunctional Analysis, Faculty of Health Sciences, Graduate School of Biomedical Sciences, The University of Tokushima, and Professor Koichi Ute of the Graduate School of Decision Science and Technology, Faculty of Decision Science and Technology, The University of Tokushima, we have developed the Hydrogel Adsorption Separation (HAS) method, a novel bioseparation method for the highly efficient, high yield, and highly purified recovery of Extracellular Vesicle (EV) from various body fluids using hydrogel beads. The Hydrogel Adsorption Separation (HAS) method, a novel bioseparation method, has been developed to recover Extracellular Vesicle (EV) from various body fluids with high efficiency, high yield, and high degree of purification using hydrogel beads.
We have been developing technologies to efficiently process body fluids such as urine and blood. In the in vitro diagnostics business, we have commercialized magnetic beads for bioseparation to extract target biomolecules from body fluids containing many foreign substances, and have accumulated a lot of know-how. By making full use of this experience and interface control technology, we have succeeded in realizing the “HAS method,” a unique principle that solves the problems of conventional bioseparation methods.
The following figure shows a schematic of the purification of Extracellular Vesicle (EV) by the HAS method. The method begins with the contact of a biological sample (blood, urine, cell culture medium, etc.) with hydrogel beads. The beads are designed to absorb the biogenic sample and swell in about 30 minutes. Microscopically, the surface of the beads is like a mesh, and during the absorption process, the liquid moves through the mesh into the beads, resulting in the separation of (1) low molecular weight foreign substances, (2) Extracellular Vesicle (EV), and (3) high molecular weight foreign substances. Low molecular weight foreign substances pass through the mesh and are absorbed into the beads. Extracellular Vesicle (EV) are trapped in the mesh because the surface of the beads is covered with an Extracellular Vesicle (EV) affinity component. Polymeric contaminants do not interact strongly with the bead surface and are washed away in the following washing process. In particular, polymeric contaminants change their conformation and zeta potential depending on the pH of the specimen, and the degree of purification may vary depending on individual specimens.
In the next step, Extracellular Vesicle (EV) adsorbed on the beads are desorbed by free solution to obtain the final purified product. Since bioseparation is completed by swelling of the beads and release of Extracellular Vesicle (EV), purification is possible in as short a time as 90 minutes, regardless of the scale.
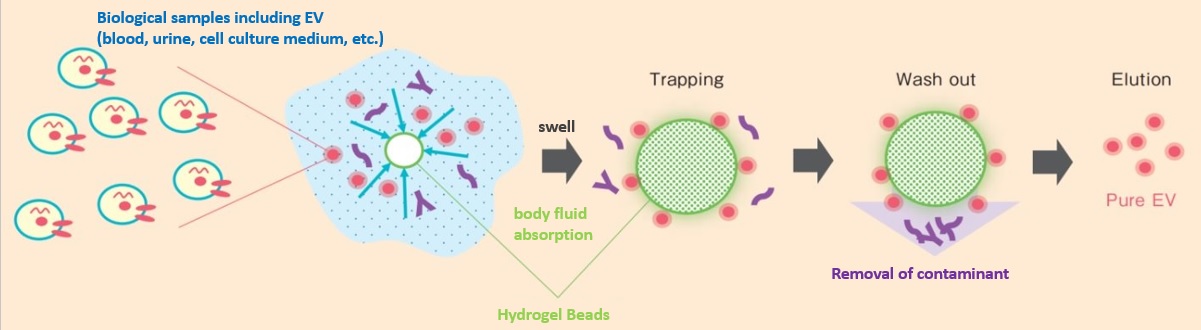
Fig. Purification of Extracellular Vesicle (EV) by the “HAS method
Standard Protocol Overview
A spin column containing hydrogel beads and the supplied Wash buffer and Elution buffer are used.
Purification is completed using a small tabletop centrifuge with 1.5-mL microtubes available.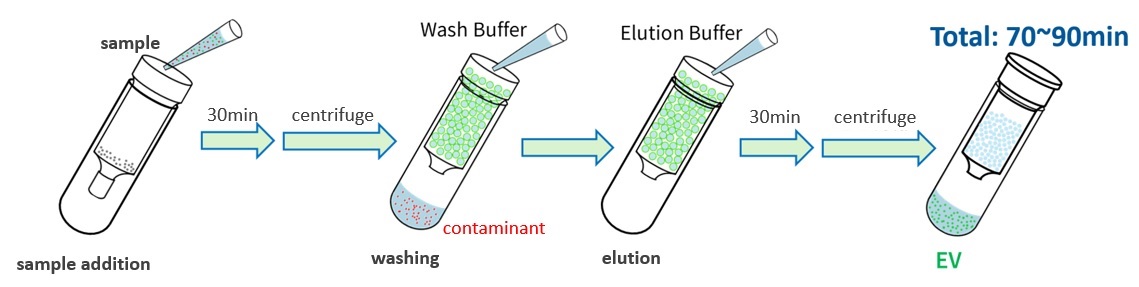
Comparison of purification methods for Extracellular Vesicle (EV)
The greatest advantage of this method is the amount of Extracellular Vesicle (EV) recovered per sample, which is more than 10 times higher than that of the gold standard ultracentrifugal method (see figure below). This feature contributes to highly sensitive detection of Extracellular Vesicle (EV) contained in extremely small amounts in body fluids, but it is also useful in drug discovery to obtain large amounts of Extracellular Vesicle (EV) for therapeutic use. Unlike some affinity purification methods, the final purified product does not contain reagent-derived chemicals and does not disrupt the lipid bilayer structure of Extracellular Vesicle (EV), which are also major advantages in drug discovery.
ELISA
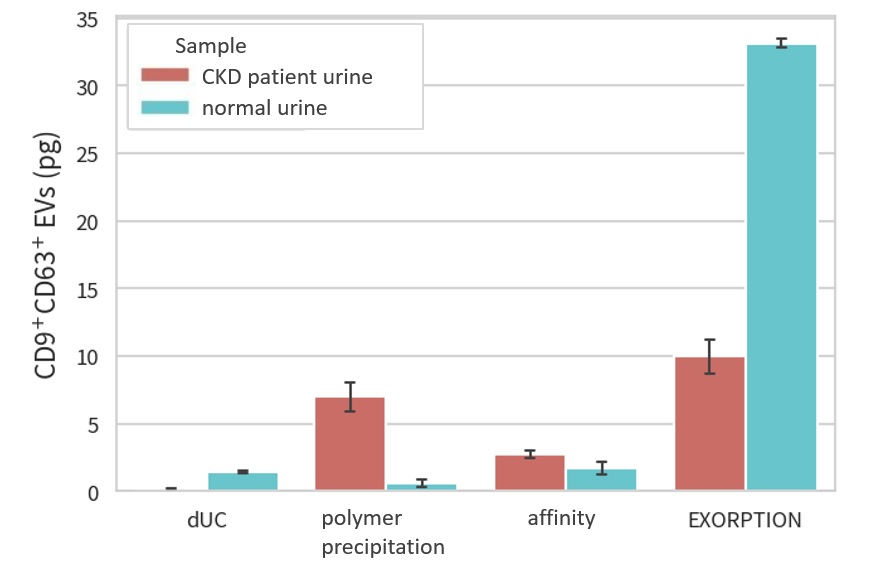
Urine specimen provided by The University of Tokushima
SDS-PAGE
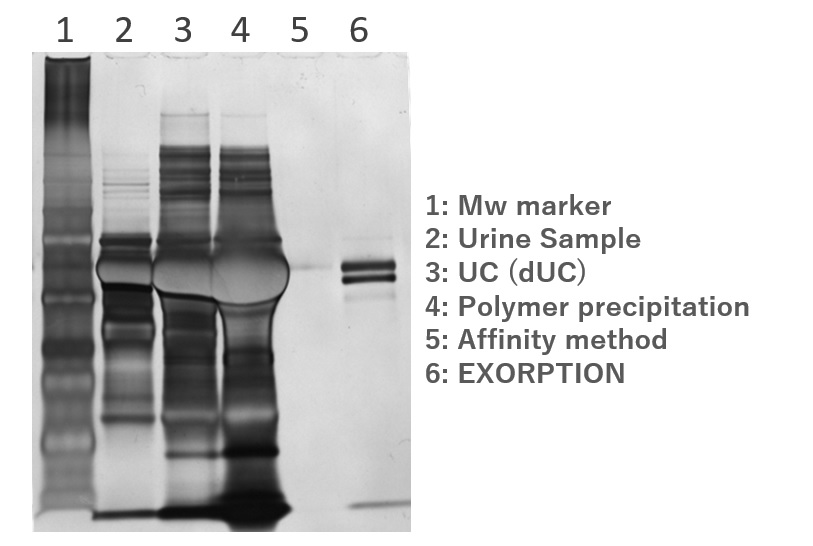
CKD (chronic kidney disease) patient urine
Examples of purification and analysis of Extracellular Vesicle (EV) in various samples using “EXORPTION”.
EXORPTION is a kit for drug discovery research that enables easy, rapid, and highly purified purification of Extracellular Vesicle. The recovered Extracellular Vesicle (EV) can be used for various analyses and experiments, including confirmation of particle count, evaluation of EV markers, nucleic acids, etc., and confirmation of physiological activity.
Purification and analysis of Extracellular Vesicle (EV) from various samples
Evaluation samples: human urine, human serum, human plasma, cell culture supernatant, etc...
List of analyses performed on refined EV
Nanoparticle tracking analysis (NTA)
EV marker detection (ELISA, Western blot)
RNA quantification, RT-qPCR
Proteome analysis
Single vesicle analysis (flow cytometer)
EXORPTION -Nanoparticle Tracking Analysis (NTA)-
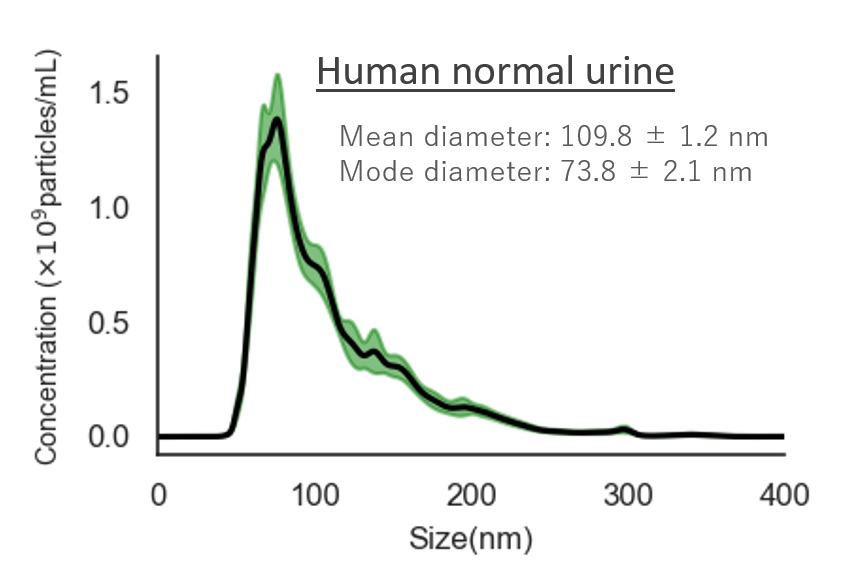
Human normal urine
Mean diameter:109.8 ± 1.2 nm
Mode diameter:73.8 ± 2.1 nm
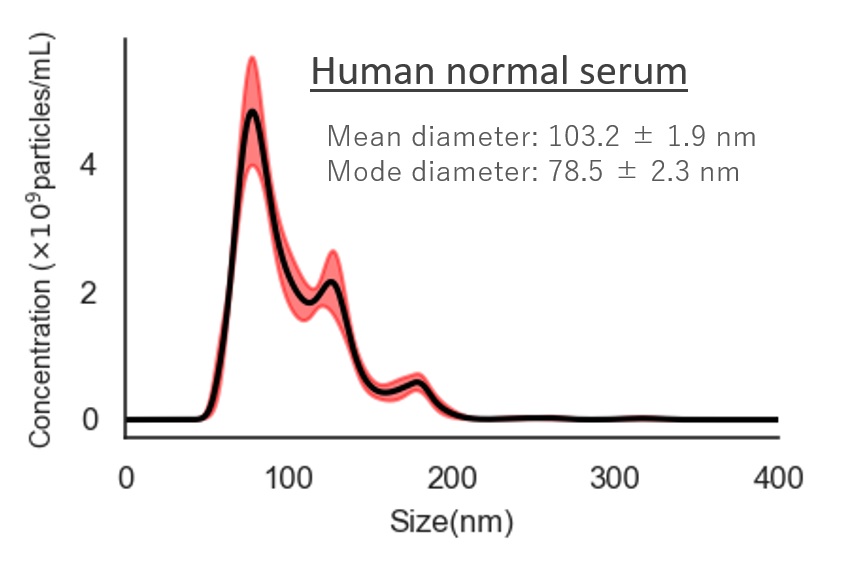
Human normal serum
Mean diameter:103.2 ± 1.9 nm
Mode diameter:78.5 ± 2.3 nm
*Purification of urine and culture supernatant from 1mL
Serum and plasma are purified from 0.2mL by dilution
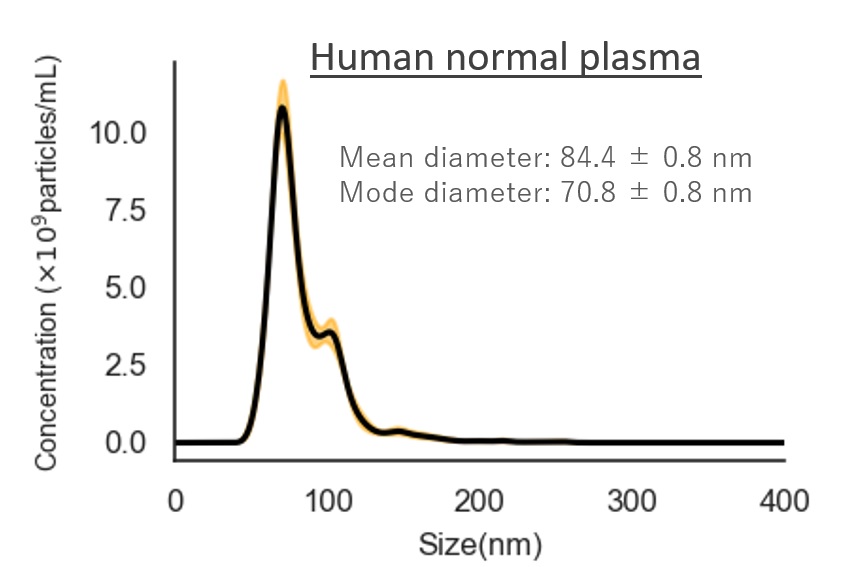
Human normal plasma
Mean diameter:84.4 ± 0.8 nm
Mode diameter:70.8 ± 0.8 nm
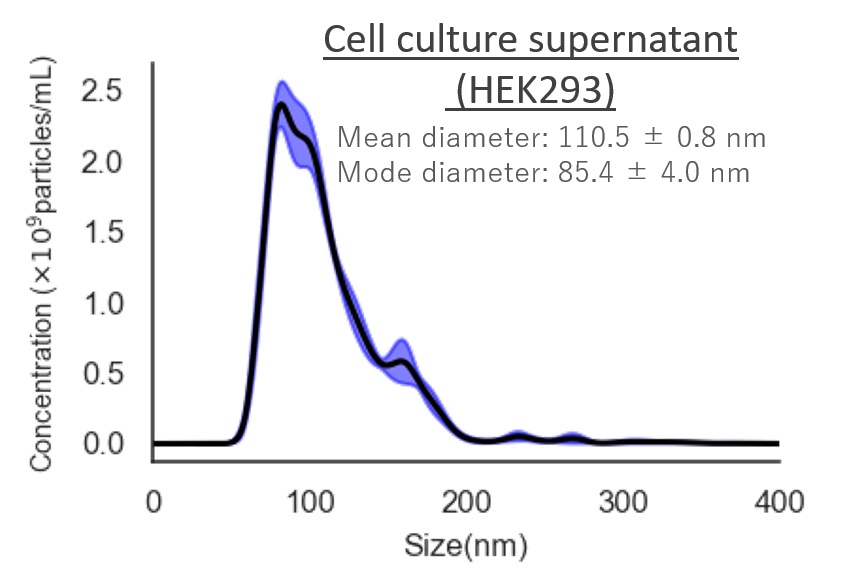
Cell culture supernatant (HEK293)
Mean diameter:110.5 ± 0.8 nm
Mode diameter:85.4 ± 4.0 nm
EXORPTION -Extracellular Vesicle (EV) purification from each sample
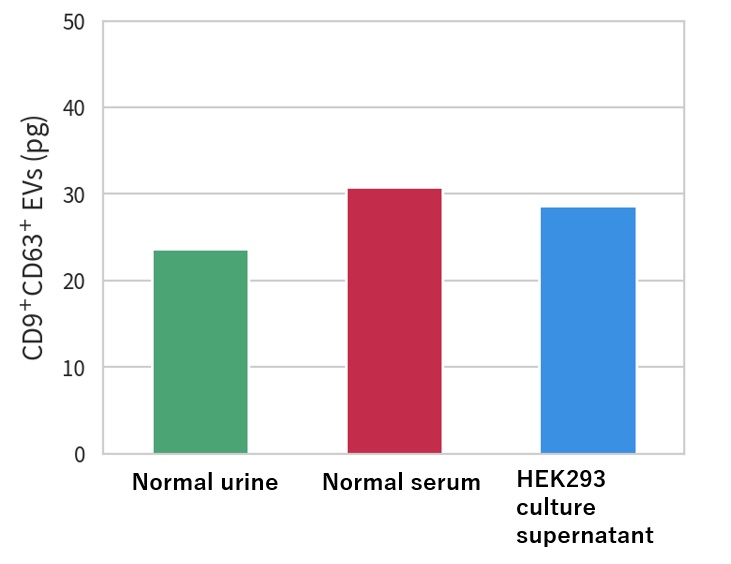
Check Extracellular Vesicle (EV) markers in each sample
→ Successful detection of Extracellular Vesicle (EV) markers regardless of the sample
Quantification of CD9+/CD63+ EV by ELISA (CD9/CD63)
Western Blot
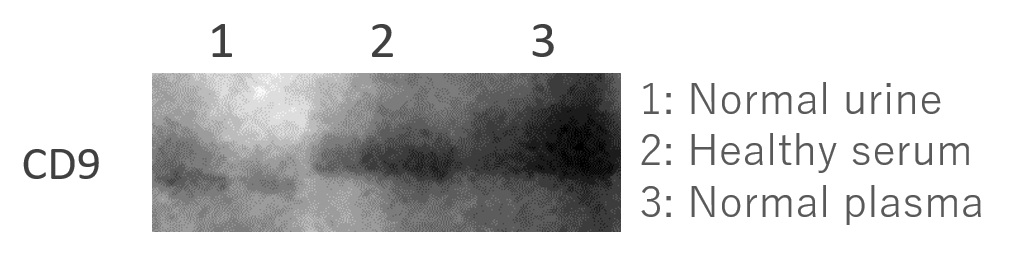
EXORPTION -Extracellular Vesicle (EV) purification from each sample
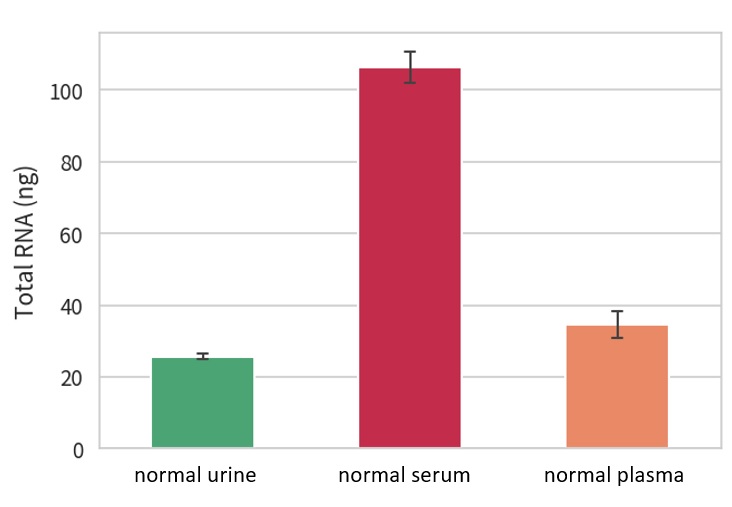
Evaluate the amount of RNA in the purified Extracellular Vesicle (EV) solution from each sample
Confirmation of the presence of miRNA in purified EV
Total RNA in EV
RT-PCR
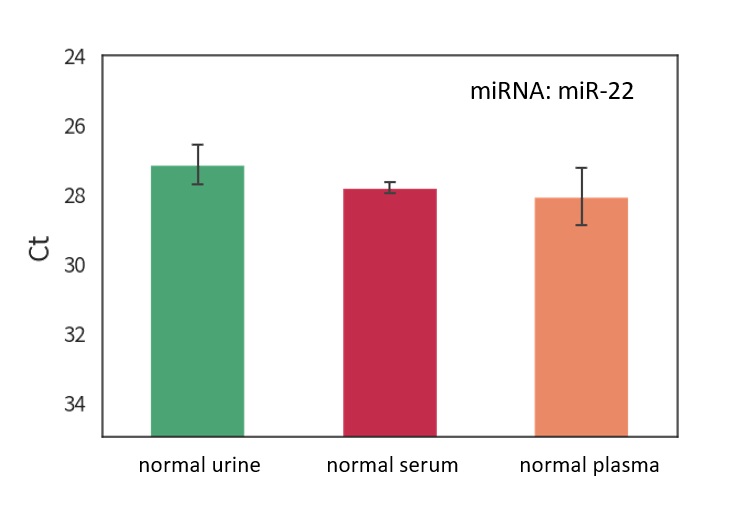
EXORPTION -Extracellular Vesicle (EV) purification from each sample
Proteome analysis results
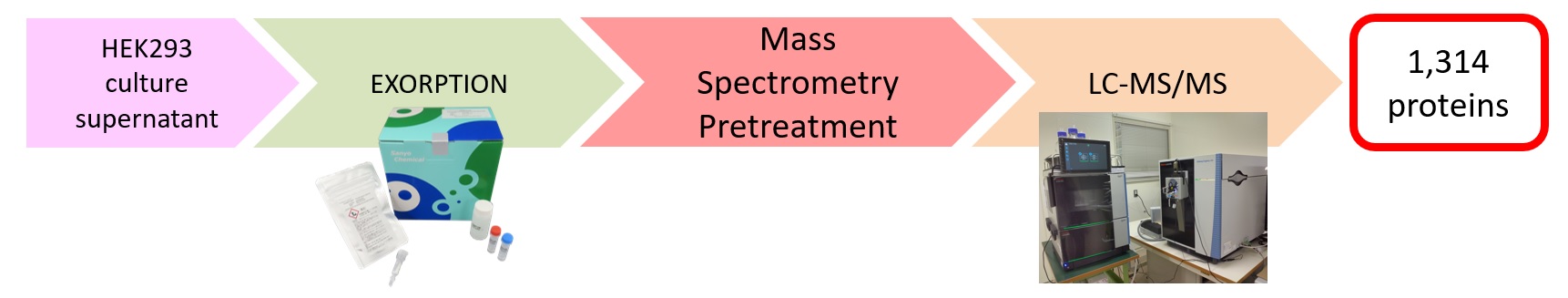
GO enrichment Analysis Results
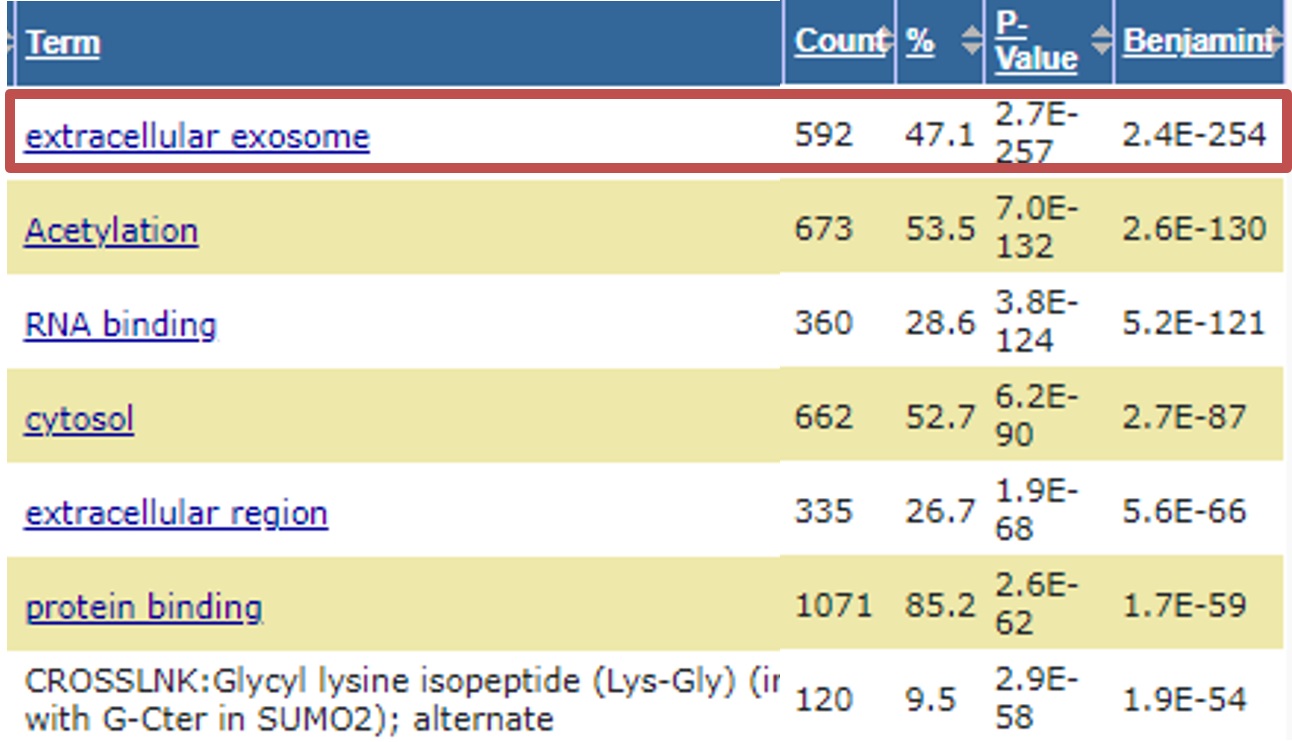
EV-derived proteins were identified from Extracellular Vesicle (EV) purified by EXORPTION
(e.g. CD9, CD63, CD81, HLA-A, HLA-B, BSG, GPC1, etc...)
Data provided by APRO SCIENCE GROUP, Pharma Foods Co.
EXORPTION -Single Vesicle Analysis
Single vesicle analysis with BD FACSymphony™ A1
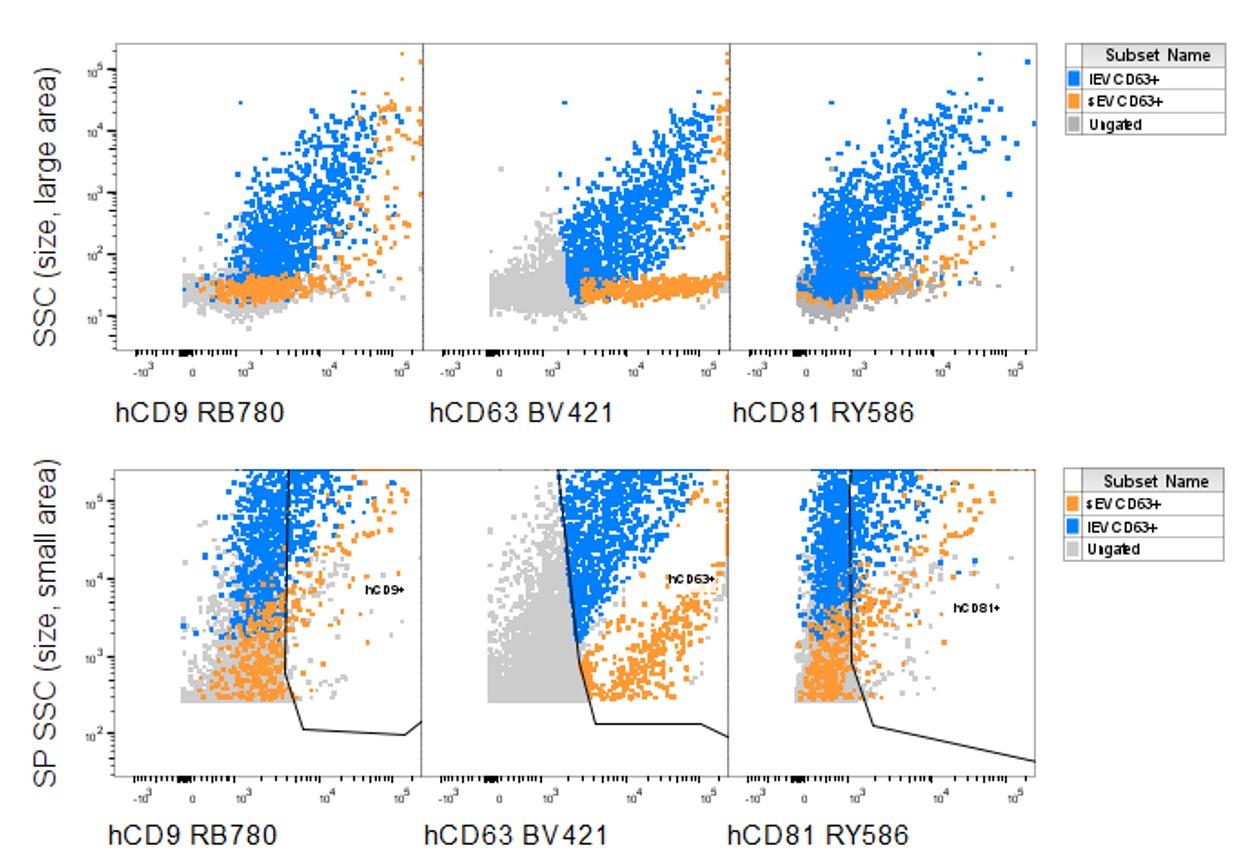
Sample: Human normal human urine
Data provided by: Nippon Becton Dickinson K.K.
Confirmation of both large EV (lEV) and small EV (sEV) from EXORPTION purified EV
lEV: CD9, CD63, CD81 all positive
sEV: CD63 positive, but CD9 and CD81 weakly positive or negative
Product introduction “EXORPTION”.

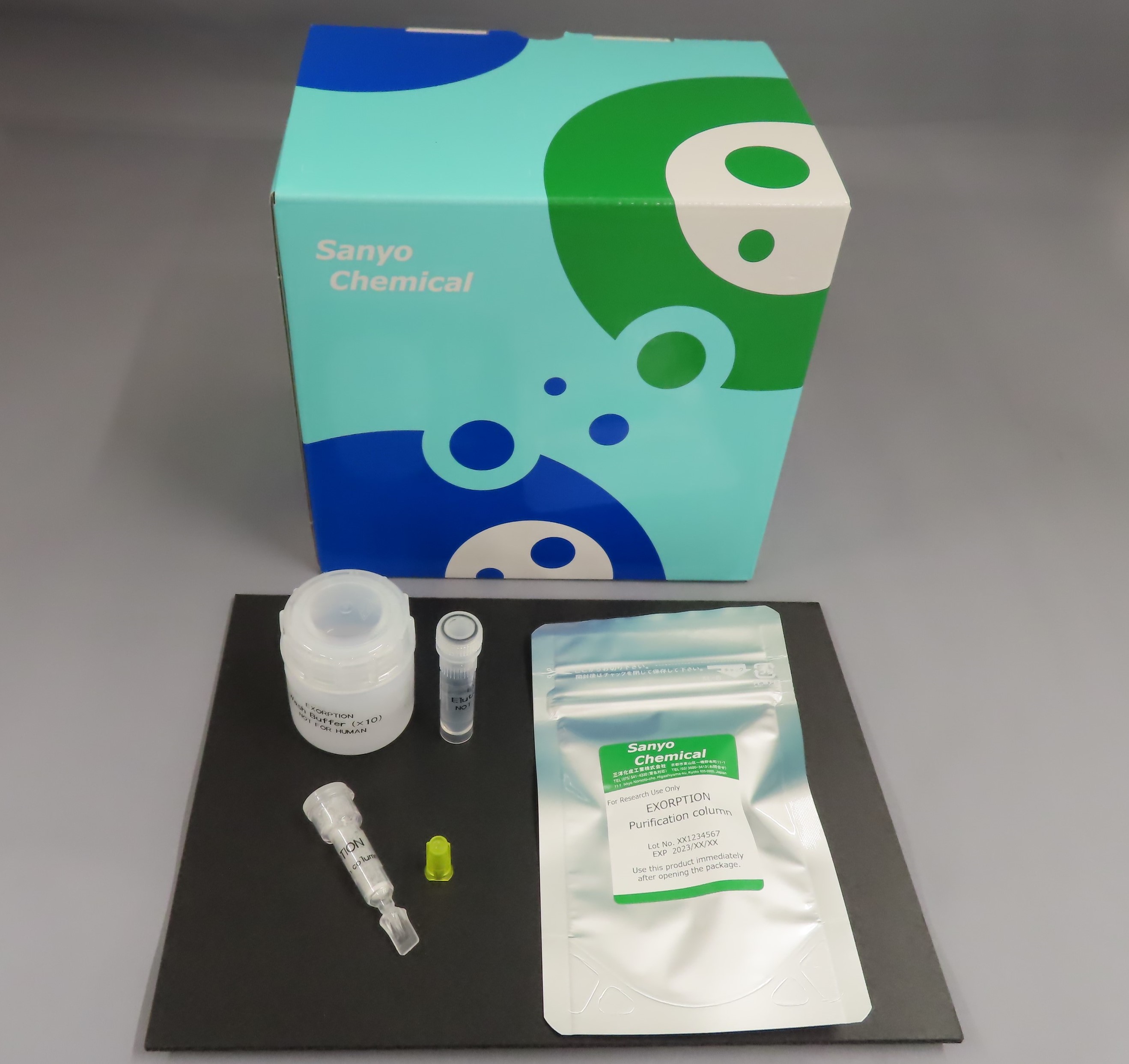
Contents
Purification column 10
LureCAP 10
Wash Buffer (×10) 1.7mL 1
Elution Buffer 1.7mL 1
Container for dilution 1
preservation
room temperature
Apparatus and equipment required in addition to this product (main items)
Micropipettes
Pipette Tips
2mL microtubes
Tabletop Centrifuge
Cooled centrifuge
Vortex mixer
*We recommend the use of low adsorption grade products for pipette tips, etc.
*This kit is intended for sale to healthcare professionals and researchers as it is a research reagent.
FAQ about “EXORPTION”.
What samples can be purified?
We have experience in purification from urine, serum, saliva, and cell culture supernatants.
How long does it take to purify with this purification kit?
Sample adsorption takes 30 minutes (static), washing takes 10 to 30 minutes, and elution takes 30 minutes (static), for a total of 90 minutes.
What do you recommend for sample pretreatment?
It is recommended to remove foreign substances in the sample by centrifugation, etc. before purification with the kit.
What is the sample volume per purification?
The standard protocol allows for purification of 1 mL of sample. If the sample volume is less than 1 mL, dilute the sample with PBS or saline to prepare 1 mL. Some protocols allow purification of up to 4.6 mL of sample if the concentration of EV in the sample is low.
What is the amount of Extracellular Vesicle (EV) recovered per purification?
Although it depends on the nature of the sample, it has been confirmed that about 20 to 150 pg of EV are recovered by quantification with the CD9/CD63 Exosome ELISA Kit, Human (Cosmo Bio, part number: EXH0102EL) and about 1010 to 1011 particles/mL by nanoparticle tracking analysis (NTA). The recovery of EV was confirmed to be about 1010 to 1011 cells/mL by nano particle tracking analysis (NTA).
Can this purification kit be reused?
This kit is not reusable.
Are there any precautions regarding the use of purified Extracellular Vesicle (EV)?
Since the purified solution contains high concentrations of inorganic salts, dilute the purified solution or perform desalting if the purified EV is used in an experiment where the presence of salt may affect the results.
As an example, the data in this document were measured using the following protocol
NTA: Dilute the purified solution with ultrapure water before measurement.
ELISA: Dilute the purified solution with 0.2 w/v % BSA solution 3 to 5 times and add to ELISA plate (in case of CD9/CD63 Exosome ELISA Kit, Human).
miRNA analysis: Use the purified solution as is (Norgen, Inc, Exosomal RNA Isolation Kit)
References
1) Report on Therapeutic Products Using Extracellular Vesicle (EV) Containing Exosomes
January 17, 2023 Scientific Committee of the Pharmaceuticals and Medical Devices Agency
This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist





