Surfactant Basics 1 (Detergents)
- What is a surfactant?
- Surfactant functions introduction video
- Surfactant's cleaning function
- Surfactant's ability to lower interfacial tension
- Cleaning and dirt potential energy
- Use of surfactants in laundry detergents
- Examples of surfactant use in kitchen detergents
- Examples of surfactant use in housing detergents
- Example of surfactant use in shampoo
- Example of surfactant use in cleaning agents for precision parts
- Related products(surfactants, detergent-related products)
What is a surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
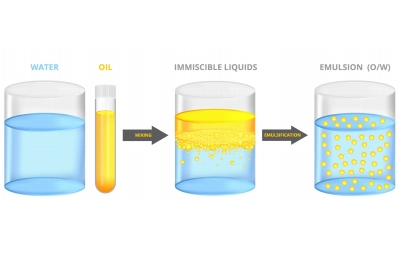
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
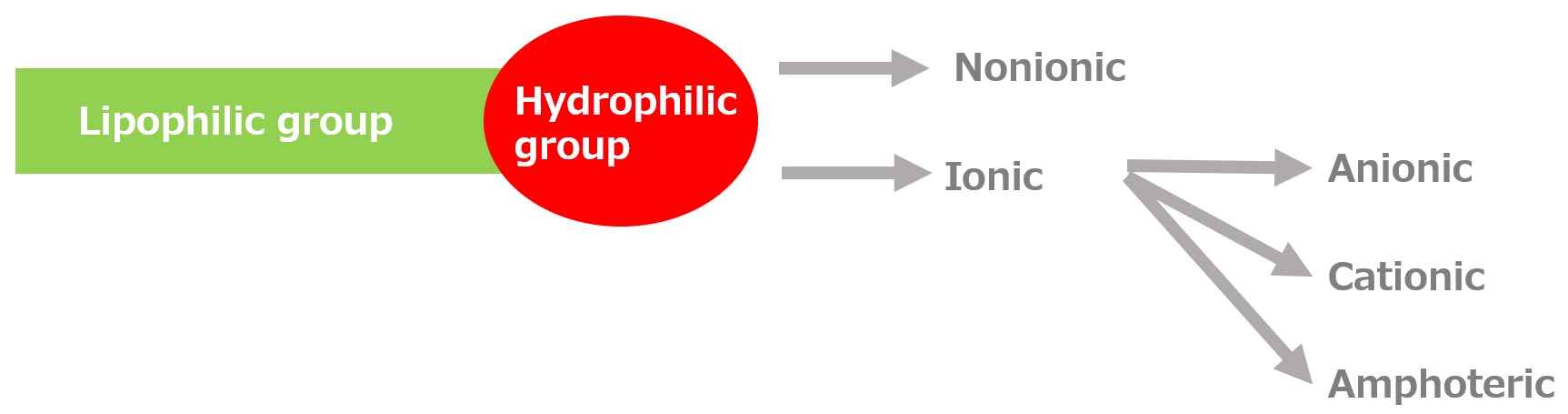
Basic structure of a surfactant
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-fitting parts) and hydrophilic groups (water-fitting parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant |
-Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH |
-Clothing detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil |
-Polyoxyethylene alkyl ether etc. |
| Anionic surfactant |
-Excellent emulsification and dispersibility -Good lather -Temperature insensitive |
-Clothing Detergent -Shampoo -Body soap |
-Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant |
-Adsorption to fibers -Antistatic effect -Bactericidal |
-Hair rinse -Fabric softener for clothes -Disinfectant |
-Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant |
-Mild on skin -Excellent solubility in water -Synergistic with other active components |
-Shampoo -Body soap -Kitchen detergent |
-Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant functions introduction video
It consists of seven short movies for each function.
0:00 Introduction of surfactants functions
0:16 Part1 Washability
1:00 Part2 Permeability
2:10 Part3 Dispersion
2:55 Part4 Foaming properties
3:25 Part5 Defoaming properties
3:39 Part6 Smoothness
4:20 Part7 Antibacterial properties
Surfactant's cleaning function
This page describes one of the typical functions of surfactants, the washing function.
The function of surfactants in washing is first to adsorb dirt. Next, it penetrates between the dirt and the clothes.
Then, it removes the stain from the garment and stabilizes it by dispersing and emulsifying the stain into small particles, thereby preventing re-contamination of the garment.
1 Adsorption
Initially, surfactant molecules adsorb onto the oily stain, making it easier to get wet.
The alkyl groups of the surfactant stick to the oily dirt and the hydrophilic groups.

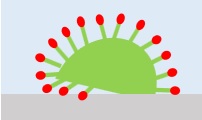
2Osmosis
The surfactant's penetrating action allows the surfactant to penetrate between dirt, fibers and other objects to be washed.
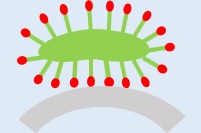
3Mechanical action
The surfactant, by penetrating between the dirt and the object being washed, helps the dirt to separate from the object when mechanical action is applied.
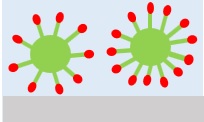
4Dispersion and emulsification
Surfactants can lower the interfacial tension between water and oil, and prevent re-contamination of clothes by dispersing and emulsifying dirt that has left the object to be washed into a smaller size in water and stabilizing it (Prevention of re-contamination ).
5Foaming action
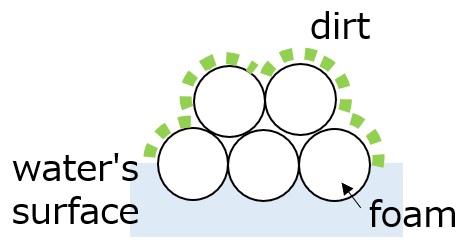
The surfactant's foaming action captures dirt on the surface of the foam generated and pulls it away from the surface of the fiber.
(Although foam has no direct relationship to cleaning power, it reduces friction during washing, catches dirt, and lifts it off the surface.)
Surfactant's ability to lower interfacial tension
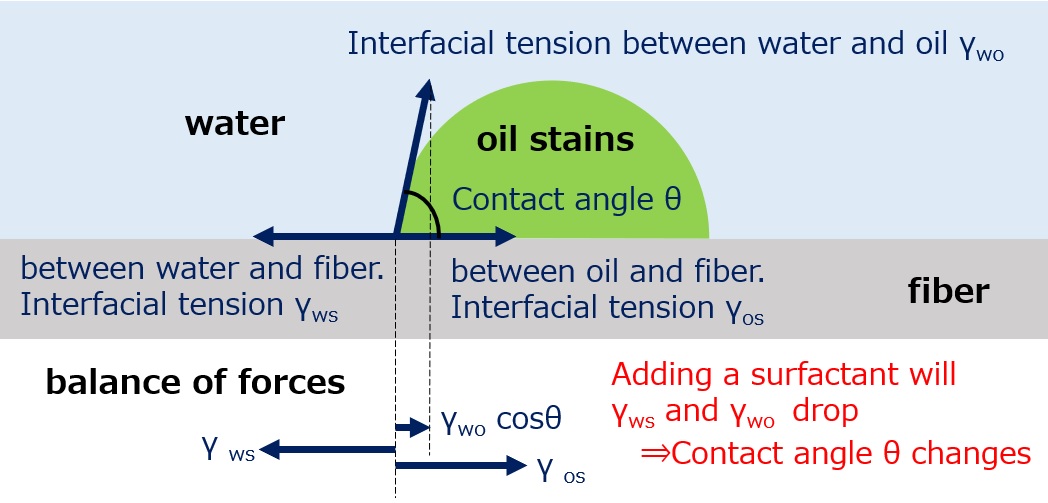
Figure: Forces acting on dirt
When an oil-stained fiber is in water, it looks like the figure when viewed from the side. At the point where the oil stain, water, and fiber come into contact, the three interfacial tensions are in balance, so the following equation can be used to express the balance of forces.
γws =γos + γwo cosθ
Next, when a surfactant is added to the water, the balance of the three forces changes as the surfactant adsorbs at the interface between water and oil stains and between water and fibers, reducing the interfacial tension (γws, γwo) of these surfaces.
When the surfactant addition reduces the interfacial tension between water and oil stain, and between water and fiber, and changes the balance of forces, the contact angle θ of the oil stain increases, and with it, the oil stain becomes rounder and finally migrates into the water. This process of transformation of oil stains is called rolling up and is one of the most important phenomena for cleaning.
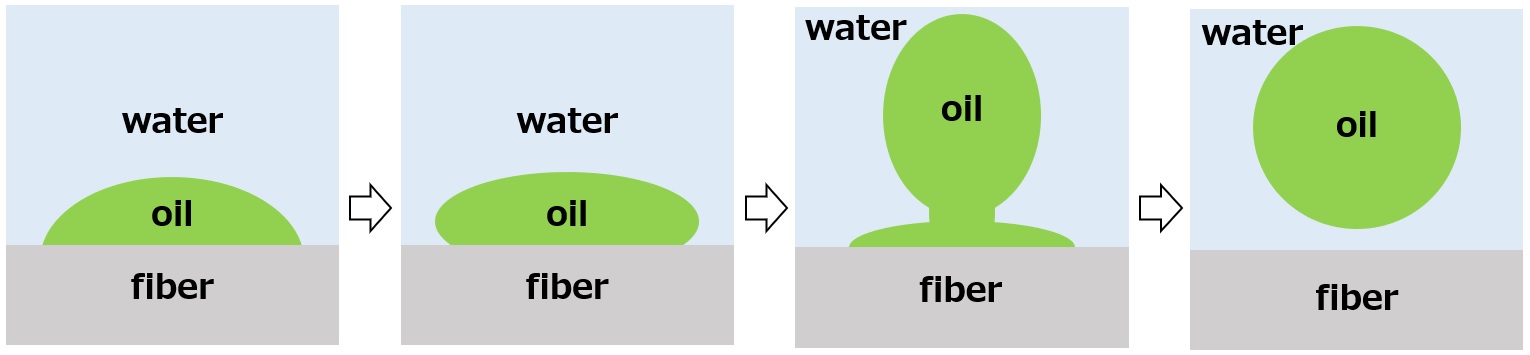
Figure: Rolling Up
Cleaning and dirt potential energy
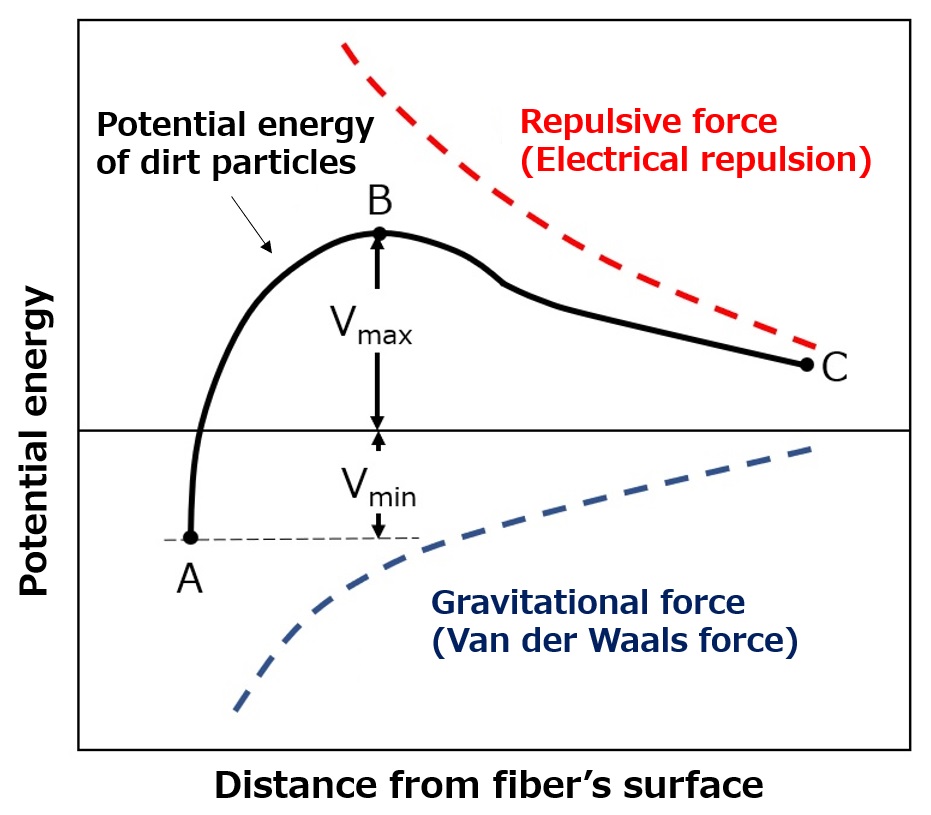
Figure: Potential energy of contamination (image)
Between dirt particles and fibers, there are forces of attraction (van der Waals force) and repulsion (electrical repulsion: both dirt particles and fibers are generally negatively charged in water), and the potential energy of dirt particles is the sum of these two types of energy.
Ease of cleaning (point A => point B => point C)
Point A is the state where dirt fibers adhere to the fiber, and in order for the dirt particles to leave the fiber, they must go beyond point B. The smaller this value is, the easier it is to clean. The difference in potential energy between points A and B (Vmax + Vmin) determines how easy it is to clean. The smaller this value is, the easier it is to clean dirt, and the larger this value is, the harder it is to clean.
Recontamination (Point C => Point B => Point A)
Point C indicates the state where dirt particles have completely left the fiber. If the difference in potential energy between points B and C is small, particles that went to point C will easily return to point A through point B (re-contamination).
Role of surfactants
Surfactants in detergents work by increasing the height of the potential energy peaks and bringing them closer to the fiber surface, making it easier to remove dirt and harder to recontaminate. Anionic surfactants are the most commonly used type of surfactant. This is because anionic surfactants work by adsorbing on the dirt and fibers to further increase the negative charge and repulsive energy (electrical repulsion).
Use of surfactants in laundry detergents
In the course of daily life, a variety of stains adhere to clothing. Dirt comes from the body (sebum, sweat, etc.) and dirt from the outside (dust, mud, etc.), and can be broadly divided into hydrophilic dirt such as sweat, blood, and fruit juice, and lipophilic dirt such as sebum, dirt, cosmetics, and cooking oil.
Clothing materials used for washing range from natural fibers such as cotton, linen, and wool to recycled fibers such as rayon, semi-synthetic fibers such as acetate, and synthetic fibers such as nylon, polyester, and acrylic. Various types of clothing detergents need to be used at home to remove the various stains on them.
Five elements of laundry that effectively remove stains
Clothing, dirt, detergent, water, and mechanical action
Examples of Ingredients for Clothing Detergent

| Surfactant | Contributes to cleaning by wetting, lowering interfacial tension, emulsifying and dispersing, and preventing recontamination. |
|---|---|
| Alkali builder | Make the cleaning solution alkaline to increase the surface potential of the fibers and dirt to increase their resilience. |
| Metal ion supplements | Surfactants and alkali builders bind to Ca and Mg ions in water, their function is reduced, so these ions are supplemented. |
| Anti-recontamination agent | Prevents reattachment of dirt once it has left the fiber. |
| Enzymes | Protein stains and grease stains are decomposed and washed off easily. |
| Other | Bleach, anti-caking agent, etc. as needed. |
Typical examples of surfactants used in clothing detergents
The actives used in laundry detergents are mainly anionic or nonionic surfactants. In actual use, these are rarely used alone, but rather in combination.
| Type | Component | Structure |
|---|---|---|
| Anionic surfactant | Sodium fatty acid (soap) | R-COO-Na+ |
| Anionic surfactant | Sodium linear alkylbenzenesulfonate (LAS) | R-C6H4SO3-Na+ |
| Anionic surfactant | Sodium alkylbenzenesulfonate (AS) | R-O-SO3-Na+ |
| Anionic surfactant | Sodium alkyl ether sulfate (AES) | R-O(CH2CH2O)SO3-Na+ |
| Anionic surfactant | Sodium alpha-olefin sulfonate (AOS) | R-CH=CHCH2SO3-Na+ |
| Nonionic surfactant | Polyoxyethylene alkyl ether (AE) | R-O(CH2CH2O)H |
Examples of surfactant use in kitchen detergents
Location of residence and type of stains
| Where to wash, what to wash | Type of stain | |
|---|---|---|
| Kitchen | tableware, vegetables around the range hood ventilation fan |
-animal and vegetable fats and oils, proteins, starches, mud, pesticide residues -modified fats and oils (fats and oils oxidized or polymerized by heat or light and turned into resin) -stains from scorching (stains such as fats, oils, and grease that have been carbonized by high heat) |
| Living room | floor (wood, carpet) walls, furniture windows, screens |
dust, mud, spills, grease, handprints, cigarette stains |
| Bathroom | bathtubs, floors, drains walls and ceilings |
soap scum (Ca salts of fatty acids, etc.), complex stains consisting of body-derived proteins and lipids |
| Toilet | toilet bowl, floor | combination of phosphoric acid, uric acid, and other bacterial metabolites with polyvalent metals in tap water. |
The main detergents used in the kitchen area include liquid kitchen detergent, cleansers, bleach, automatic dishwasher detergent, and range hood/exhaust fan detergent.
Examples of Ingredients for Kitchen Cleaning Agents

| Surfactant | Alkyl ether sulfates are commonly used because of their superior cleaning power, foaming ability, and solubility, and are generally used because they cause less hand irritation. |
|---|---|
| Foam stabilizer/thickeners | Diethanolamide of fatty acids obtained from coconut oil or palm kernel oil, are often used because of their foam stabilizing effect and thickening effect in blended systems. |
| Anti-Hand Roughness Agent | Amine oxide and amide betaine-type amphoteric surfactants are known to prevent rough hands. |
| Solubilizer | It is added so that kitchen detergents do not harden or become cloudy in winter. |
Examples of surfactant use in housing detergents
Detergent for living room
The majority of dirt in living rooms is dirt applied by mud, textile dust, and limbs. Most interior materials used in homes are surface-treated with synthetic resins. These materials are lipophilic, which means that they are prone to oily stains, and they are generally electrostatic, which means that dust and cigarette smoke can easily stick to the surface.
Most of the dirt and dust can be removed with an electric vacuum cleaner, but for the dirt that cannot be removed, detergents are required. Typical detergents used for living room stain removal include wood floors, general-purpose detergents for furniture, carpet detergents, and window cleaner.
Detergent for wood floors, walls, and furniture
In addition to wood, the target is stains on floors, walls, and furniture tatami mats made of new construction materials. The stains are usually composed of dirt from hands and feet, dirt and dust, tobacco tar, etc., and can be removed relatively easily by the action of surfactants.
Therefore, detergents that mainly use surfactants are effective, including concentrated types that are diluted with water and low-concentration types that can be used as is and do not require wiping twice. However, all of them require a trial wipe once before use to make sure that they will not damage the surface of white wood or painted surfaces.
Examples of detergent formulations for wood floors, walls, and furniture
| Surfactants (e.g., polyoxyethylene alkyl ethers) | 0.5 to 1% |
| Water and water-soluble organic solvents (e.g., isopropyl alcohol) | remaining quantity |
| Total amount | 100 |
Carpet cleaner
There are two types of carpet cleaning: whole washing and partial washing. In the case of whole washing, detergents similar to those used for washing clothes can be used. Stains that can be partially washed are mainly stains such as food spills. Carpets require a long drying time if water is used, so a washing method in which the stain is powdered with detergent and sucked up with an electric vacuum cleaner is suitable.
Aerosol and powder detergents are available, which are generally composed of surfactants, alkalis, solvents, and adsorbent powders.
-Aerosol type detergents are made up of an aqueous solution of surfactant, water-soluble organic solvent, alkali, and adsorbent powder, so they absorb dirt and dry.
-The powder type is a combination of an adsorbent powder such as urea resin, a surfactant, and a solvent, which is sprayed on the stain and brushed to absorb the stain.
Example of carpet cleaner (powder type) formulation
| Adsorbent powder (e.g., urea resin powder) | Approx. 60 |
| Surfactants (e.g., polyoxyethylene alkyl ethers) | Approx. 5 |
| Water and water-soluble organic solvents (e.g., isopropyl alcohol) | remaining quantity |
| Total amount | 100 |
Detergent for window glass
Window stains are usually composed of a complex of water-soluble and non-water-soluble components.Therefore, water alone does not easily remove stains, requiring the action of surfactants, alkalis, and water-soluble organic solvents.
Window cleaning is often performed on poor footholds, and it is desirable to wipe windows less frequently by hand. Therefore, the mainstream window cleaner is an aerosol or spray cleaner that can be sprayed on and then wiped off in a single pass. Also, since window glass is almost always a vertical surface, it is necessary for the detergent solution to remain on the glass surface for a while. For this reason, the detergent is sprayed in the form of bubbles to prevent streaks.
Surfactants commonly used in window glass detergents include linear alkyl benzene sulfonate amine salts, alkyl ether sulfate amine salts, and polyoxyethylene alkyl ethers. Note that the concentration of surfactants is high, which tends to leave streaks on glass surfaces, so the ratio of surfactants is kept low.
Example of window cleaner formulation
| Surfactants (e.g., linear dodecylbenzene sulfonate amine salts) | 0.1% |
| triethanolamine | small quantity |
| Water and water-soluble organic solvents (e.g., isopropyl alcohol) | remaining quantity |
| Total amount | 100 |
Bathroom Detergent
The areas and stains to be cleaned in the bathroom are (1) hot water stains in the bathtub and (2) mildew on the soot and walls. Below are examples of detergents used to remove each of these stains, their functions, and formulations.
Detergent for removing bathtub stains
Soap scum is a complex of soap scum and proteins and fats from the human body that adhere to the bathtub and the pipes connecting the bathtub and tub.
Soap dregs are called water-insoluble metallic soap, which is formed when alkali metal salts of fatty acids (such as Na), the main ingredient of soap, react with polyvalent metal ions such as Ca and Mg in the water.
The following detergents are used for cleaning bathrooms, depending on the degree of soiling and the material of the bathtub.
(1) Weak alkaline type
These products are designed to remove stains with the power of weak alkali and surfactants, and are intended for light stain removal.
Surfactants are required to have good penetration and cleaning power, as well as good resistance to hard water. Sodium alkyl ether sulfates, sodium linear alkyl benzene sulfonate, and polyoxyethylene alkyl ethers are used. As an alkali. Mild ones such as alkanolamine are used in the formulation.
Example of bathtub scum remover (mildly alkaline type) formulation
| Surfactants (e.g., polyoxyethylene alkyl ethers) | 10% |
| triethanolamine | small quantity |
| Water and water-soluble organic solvents (e.g., isopropyl alcohol) | remaining quantity |
| Total amount | 100% |
(2) Weak acidic type
This type of detergent targets stains mainly composed of soap scum, which is acidic, easily decomposed, and soluble in organic solvents. This type of detergent contains organic acids such as citric acid and malic acid that are acidic enough not to damage bathtub materials, water-soluble organic solvents such as propylene glycol and butylcarbitol, and a surfactant to improve cleaning power.
The function required of surfactants here is good foaming, detergency, and rinsability, even when used in combination with organic acids. Polyoxyethylene alkyl ethers and sodium linear alkylbenzenesulfonate are used alone or in combination.
Example of bathtub scum remover (mild acidic type) formulation
| Surfactants (e.g., polyoxyethylene alkyl ethers) | 10% |
| Organic acids (e.g., malic acid) | small quantity |
| Water and water-soluble organic solvents (e.g., isopropyl alcohol) | remaining quantity |
| Total amount | 100% |
(3) Cleanser type
It is used to polish and remove stains from bathtubs. Depending on the material of the bathtub, it may be scratched and lose its luster, so care should be taken. The same type of cleanser is used as that used in the kitchen.
Mold Remover Detergent
Bathrooms are high in both temperature and humidity, making them a prime breeding ground for mold and mildew. Red mold and black mold are especially fond of growing on the joints of tiles and slats used for floors and walls.
Mold can hardly be removed by washing with detergent or scrubbing with cleanser. For this reason, specialized mold remover detergents have been developed.
The main ingredient in mainstream mold removers is sodium hypochlorite, which kills mold by its oxidizing power and also decomposes and bleaches mold pigments, making them colorless. Sodium hypochlorite alone is unstable and decomposes during storage, causing it to lose its efficacy, so sodium hydroxide is added as a stabilizing agent.
Since mold remover detergent may cause damage to the human body if it gets into the eyes, gets on the skin, or is inhaled, the nozzle of the container is designed so that it does not spray out as a mist. Surfactants are added to form a moderately sticky foam and to provide cleaning power.
Surfactants must be stable under strong alkaline conditions and not impair the stability of sodium hypochlorite, and fatty acid soaps, sodium alkyl diphenyl ether disulfonates, sodium alkyl phenyl ether sulfates, and amine oxides are used alone or in combination. Amine oxide, etc. are used alone or in combination.
Example of mold remover detergent formulation
| Sodium hypochlorite | Approx. 5% |
| Sodium hydroxide | Approx. 2% |
| Surfactants (e.g., sodium alkyl diphenyl ether disulfonate) | 1 to 2 % |
| Water | remaining quantity |
| Total amount | 100% |
Toilet Detergent
Toilet stains are mainly a combination of phosphoric acid, uric acid, and other substances derived from excreta, bacterial metabolites, and Ca and iron in tap water.
Various detergents have been developed for different purposes as follows. For the purpose of actively removing stains, there are acidic types and alkaline types (chlorine-based).The acidic type has the function of dissolving stains with acid, with hydrochloric acid as the main ingredient and a cationic surfactant as a supplementary surfactant.
Alkaline types, on the other hand, are similar to bathroom mildew removers in that they decompose stains through oxidation. Since this type of detergent generates chlorine gas that is harmful to the human body when mixed with acidic substances, the "Household Goods Quality Labeling Law" requires the labeling of "Do not mix" for each type of detergent.
Example of toilet detergent (acidic type) formulation
| hydrochloric acid | 9% |
| Surfactants (e.g., lauryl dimethyl benzyl ammonium chloride) | 1% |
| water | remaining quantity |
| total amount | 100% |
In order to prevent toilet bowl stains and provide a pleasant smell, a type of toilet bowl stain prevention detergent has been developed that is dissolved in the flushing water to clean and add fragrance the toilet bowl each time it is flushed.
While the aforementioned toilet detergents are used when cleaning the toilet, these detergents are used constantly when using the toilet. There are two types: an in-tank type that is thrown into the water tank of the flushing toilet, and an on-tank type that is placed on top of the tank where the supply water is applied.
In both cases, the key point is that they dissolve gradually in water, and special polyalkylene glycol is mainly used as the base agent.
Example of detergent formulation for toilet bowl stain prevention
| Slow-dissolving base with detergent properties (e.g., special polyalkylene glycols) | Approx. 60% |
| Inorganic salt (e.g., sodium sulfate) | 5 to 30% |
| dyes | 4 to 5% |
| fragrance | small quantity |
| total amount | 100% |
Example of surfactant use in shampoo
Shampoo is classified as a cosmetic, and is defined as a hair-washing cosmetic used to cleanse the scalp and hair, control dandruff and itching, and keep the scalp and hair clean and beautiful. The product must have adequate cleansing power to remove dirt but not too much sebum necessary for the scalp and hair, and must be safe for the scalp, hair, and eyes.
In addition, since the product is discharged as wastewater, biodegradability is also an important factor, as it is easily decomposed by activated sludge treatment and microorganisms in the natural world.
Typical example of surfactants used in shampoo
| Classification | Component | Summary |
|---|---|---|
| Anionic surfactant | Alkyl sulfates (AS) Alkyl ether sulfates (AES) |
General purpose anionic surfactant. Often used in combination with hypoallergenic surfactants. |
| Anionic surfactant | Acylmethyl taurate (AMT) | With a structure similar to taurocholic acid, a biological surfactant, it has excellent safety. |
| Anionic surfactant | Alkyl ether carboxylates (ECA) | Low skin irritancy and similar structure to soap, it is also biodegradable. |
| Anionic surfactant | Alkyl ether sulfosuccinate (SS) | Low skin irritation and good foaming. Easily hydrolyzed due to the presence of intramolecular ester bonds. It should be handled in the neutral to slightly acidic pH range. |
| Anionic surfactant | N-Acylglutamate (AG) | Amino acid surfactant produced from amino acids. Lighter foam quality than AS and AES. Low irritation to skin and eyes. |
| Amphoteric surfactant | Alkyl betaine (AB) Alkylamidopropyl betaine (APB) |
General-purpose amphoteric surfactant |
| Amphoteric surfactant | Alkyl imidazolinium betaine (AIB) | Low irritation, especially to the eyes |
| Amphoteric surfactant | Alkyl aminopropionates (APL) | Amino acid type amphoteric surfactant. Easily thickened in combination with anionic surfactants. |
Example of surfactant use in cleaning agents for precision parts
In the industrial field, there are a multitude of different cleaning targets. For example, in the automotive industry, there are a vast number of parts, each of which has a different shape, size, material, and contamination composition. Furthermore, the level of cleanliness required after cleaning differs depending on the application of the parts. The same can be said for cleaning in the electrical, electronics, precision machinery, heat treatment, and plating industries.
Examples of cleaning in various industrial fields
| Industrial Fields | object to be cleaned | Cleaning purpose |
|---|---|---|
| Automotive industry | Metal fabricated parts, automobile bodies | Degreasing, removal of buffing compound, pre-paint treatment |
| Electrical and electronics industry | Printed circuit boards, semiconductor materials, electric motor materials, metalworking parts | Flux removal, degreasing, pre-paint treatment |
| Precision machinery industry | Watches, photographic machine parts, bearings | Degreasing, particulate removal |
| Heat Treatment Industry | Metalworking parts, powder metallurgy parts | Removal of fat |
| Plating industry | Metalworking parts, resin processing parts | Degreasing, removal of buffing compound |
Type of stain
| Organic material | Heat treatment oil, grease, drawing oil, cutting oil, press oil, rust inhibiting oil, and various other lubricants |
| Inorganic material | Machining scraps, burrs, abrasives, dust, rust |
| Other | Inks, fluxes, waxes, adhesives, etc. |
Type of cleaning agents
Cleaners that do not contain halogens are classified as aqueous, quasi-aqueous, or non-aqueous cleaners, as shown in the table below.
Water-based cleaning agents
Water-based cleaning agents are further classified as alkaline, neutral, and acidic.
Alkaline cleaning agents, which are typical water-based cleaning agents, consist of surfactants, builders, antifoam agents, chelating agents, and rust inhibitors. Surfactants contribute to cleaning by wetting, lowering interfacial tension, emulsifying, dispersing, and solubilizing.
Semi-aqueous cleaning agents
The components of quasi-aqueous detergents are often solvents plus water, and they are effective in cleaning oil-based stains for which water-based detergents do not have sufficient cleaning power. Solvents used as quasi-aqueous cleaning agents are glycol ethers and terpenes, and these components plus water and surfactants are used as cleaning agents. Solvents such as glycol ethers have a relatively high flash point, which can be eliminated by adding 5-20% water, and are often classified as non-hazardous under the Fire Service Law.
Non-aqueous cleaning agents
Nonaqueous cleaners are classified as hydrocarbon-based, alcohol-based, and silicone-based.
Hydrocarbon-based Cleaning Agents
Hydrocarbon-based cleaners are effective against oil-based stains. The main ingredients are normal paraffin, isoparaffin, and naphthenic hydrocarbons.
Hydrocarbon-based cleaning agents are less corrosive to metals and can be recycled by distillation, making them economical. However, since they are flammable, the cleaning equipment must be explosion-proof.
Alcohol-based Cleaning Agents
Isopropyl alcohol and ethyl alcohol are representative alcohol-based cleaning agents. Although they do not have high solubility in oil, they exhibit excellent cleaning power against water-soluble stains. They dry well and can be used as draining agents, but they have a low flash point and require adequate explosion-proof construction for cleaning equipment.
Classification Table of Cleaning Agents
| Broad category | Middle class | Subclass |
|---|---|---|
| Water system | Water | Pure water, deoxygenated water, tap water |
| Alkaline Neutral system Acidic system |
Surfactants, Alkali Builders and Water Surfactants and Water Surfactants, Acids and Water |
|
| Quasi-aqueous system | Quasi-aqueous system | Glycol ethers and water Terpenes, surfactants and water Silicones and Surfactants |
| Non-aqueous | Hydrocarbon-based Alcohol-based Silicone-based |
Naphthene, isoparaffin Isopropyl alcohol, ethyl alcohol Low molecular weight polydimethylsiloxane |
Cleaning Method
In industrial cleaning, the shapes and sizes of the objects to be cleaned are varied, and various methods are devised depending on the purpose and cleanliness, not to mention the selection of the most suitable cleaning agent. In general, the following physical methods are incorporated, followed by rinsing and drying processes as necessary to complete the cleaning process.
Cleaning in each industry must achieve the target level of cleanliness or quality problems will occur, so cleaning experiments, including cleaning equipment, are conducted repeatedly in advance.
| Ultrasonic cleaning | A method of removing dirt from the surface of objects to be cleaned by the cavitation and micro-vibration effects produced by ultrasonic waves. |
|---|---|
| Shower cleaning | A cleaning method in which liquid jetted from a nozzle under pressure is applied to the object to be cleaned. This cleaning method is more efficient than the soaking method or the ejection method. However, this cleaning method tends to foam easily, and it is necessary to select a low-foaming cleaning agent. |
| Sweep and wash | Method to remove dirt by immersing the object to be cleaned in the liquid and rocking it up and down, left and right. |
| Soak washing | A cleaning method in which the object to be cleaned is soaked in a cleaning agent to remove stains. A method that uses the dissolving and penetrating power of the cleaning solution to remove stains. |
| Jet cleaning | A method of removing dirt by a stream of cleaning agent sprayed from above, below, right and left onto the object to be cleaned immersed in the liquid. |
Related products(surfactants, detergent-related products)
As a surfactant manufacturer, we offer a lineup of numerous surfactants and detergent-related products.
Surfactants for laundry detergents and kitchen detergents

- Nonionic surfactant
Polyoxyalkylene alkylamine-based surfactant "PUREMEEL EP-300S"
Base detergent for clothes with excellent cleaning power against grease and oil stains

- Nonionic surfactant
Low-foaming nonionic surfactant for machine and metal cleaning "SEDORAN FF"
Low foaming and good foam breakability make it suitable for machine and metal cleaning by spraying or jet washing.


- Nonionic surfactant
Polyoxyethylene - Polyoxypropylene Block Copolymer ”NEWPOL PE”
Lineup of products with distinctive features and a wide variety of functions can be added

- Nonionic surfactant
Nonionic Surfactants Catalog
A list of properties of Sanyo Chemical's representative nonionic surfactants.
Surfactants for cosmetics

- Cosmetic raw materials
- Anionic surfactant
Anionic Surfactant for Cosmetics with Excellent Foaming Properties"BEAULIGHT® SHAA"
"BEAULIGHT® SHAA" has a high foaming speed and forms a fine lather. As a hypoallergenic ingredient, it is suitable as a base material for shampoos, body soaps, etc.

- Cosmetic raw materials
- Anionic surfactant
Biodegradable Ether Carboxylic Acid-based Hypoallergenic Detergent Base"BEAULIGHT® LCA-25N"
As a hypoallergenic detergent, "BEAULIGHT® LCA-25N" is suitable for sulfate-free shampoos, body soaps, etc.

- Cosmetic raw materials
- Amphoteric surfactant
Amino Acid Type Highly Functional Amphoteric Surfactant for Shampoo "PIUSERIA® AMC"
"PIUSERIA® AMC" prevents itching and dandruff by reducing the amount of surfactant residue on the skin during shampooing.
Related topics

- What is a surfactant?
- Surfactant functions introduction video
- Surfactant's cleaning function
- Surfactant's ability to lower interfacial tension
- Cleaning and dirt potential energy
- Use of surfactants in laundry detergents
- Examples of surfactant use in kitchen detergents
- Examples of surfactant use in housing detergents
- Example of surfactant use in shampoo
- Example of surfactant use in cleaning agents for precision parts
- Related products(surfactants, detergent-related products)
This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.





