Surfactant Basics 2 (Emulsion, Emulsifiers)
What is a Surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
Basic structure of a surfactant
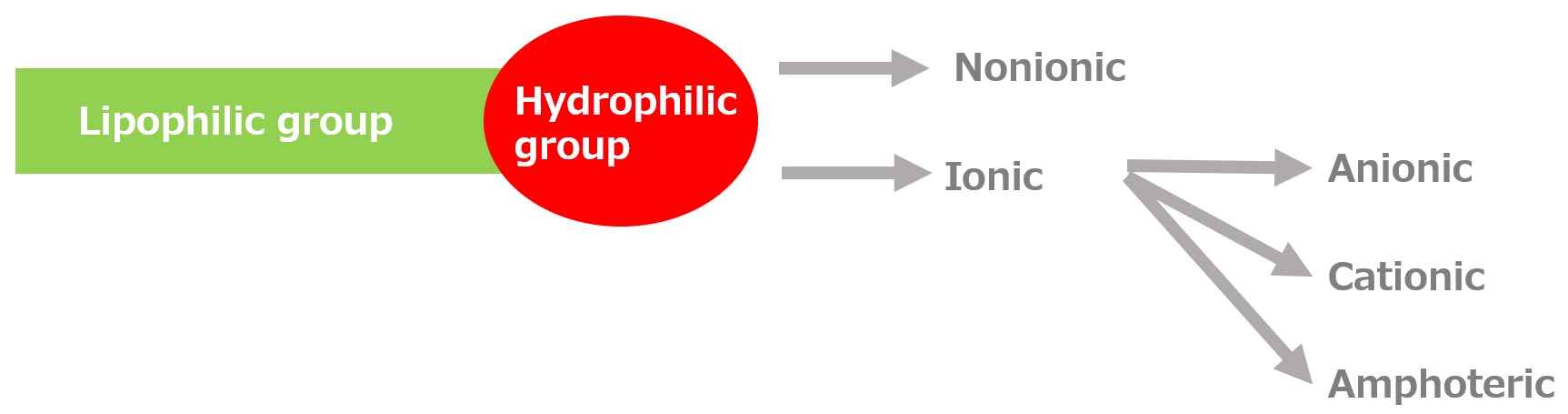
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-fitting parts) and hydrophilic groups (water-fitting parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant |
-Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH |
-Clothing detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil |
-Polyoxyethylene alkyl ether etc. |
| Anionic surfactant |
-Excellent emulsification and dispersibility -Good lather -Temperature insensitive |
-Clothing Detergent -Shampoo -Body soap |
-Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant |
-Adsorption to fibers -Antistatic effect -Bactericidal |
-Hair rinse -Fabric softener for clothes -Disinfectant |
-Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant |
-Mild on skin -Excellent solubility in water -Synergistic with other active components |
-Shampoo -Body soap -Kitchen detergent |
-Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant Functions Introduction Video
It consists of seven short movies for each function.
0:00 Introduction of surfactants functions
0:16 Part1 Washability
1:00 Part2 Permeability
2:10 Part3 Dispersion
2:55 Part4 Foaming porperties
3:25 Part5 Defoaming properties
3:39 Part6 Smoothness
4:20 Part7 Antibacterial properties
About Emulsions
What is Emulsification?
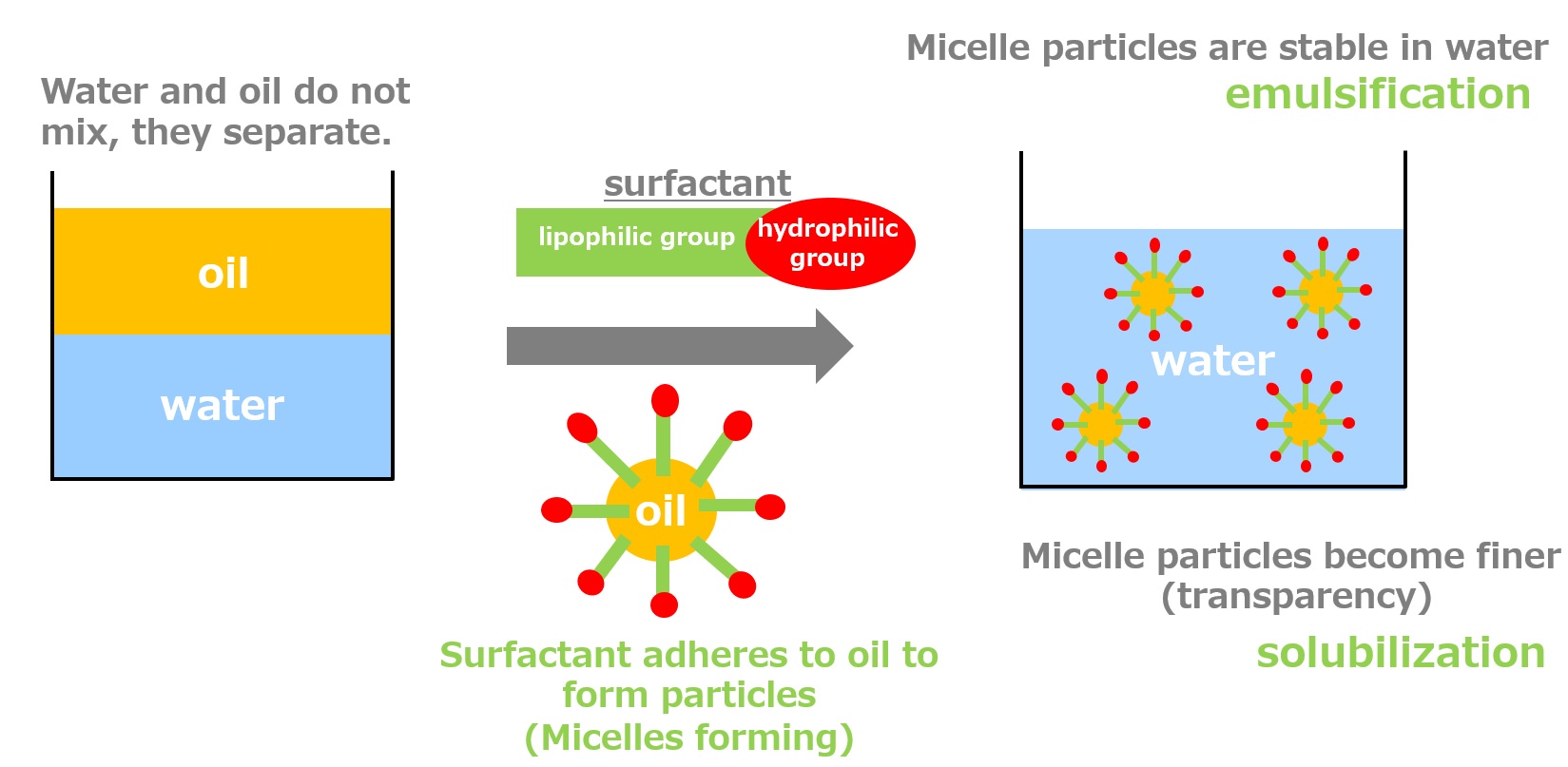
Mixing water and oil is called emulsifying, and the resulting emulsion is called an "emulsion" or "emulsion.
Water and oil do not mix and separate, but by adding a surfactant, the surfactant adheres to the oil or water and forms particles. Emulsification occurs when micelle particles are stabilized in water. Among emulsification, the phenomenon in which oil becomes transparent and mixes with water is called "solubilization. (In the solubilized state, the micelle particles appear transparent because their diameter is shorter than the wavelength of light.)
Distinction between emulsification and dispersion
A liquid (water) in which other liquids exist as fine particles is called an emulsion, and a solid in which solids exist as fine particles is called a dispersion (or suspension). In addition, emulsions and dispersions are usually distinguished according to whether they are in a liquid or solid state when mixed with water.
For example, when a liquid monomer is polymerized into fine particles in water to form solid polymer particles, this is also considered an emulsion (emulsion polymerization).
Types of emulsions (emulsions)
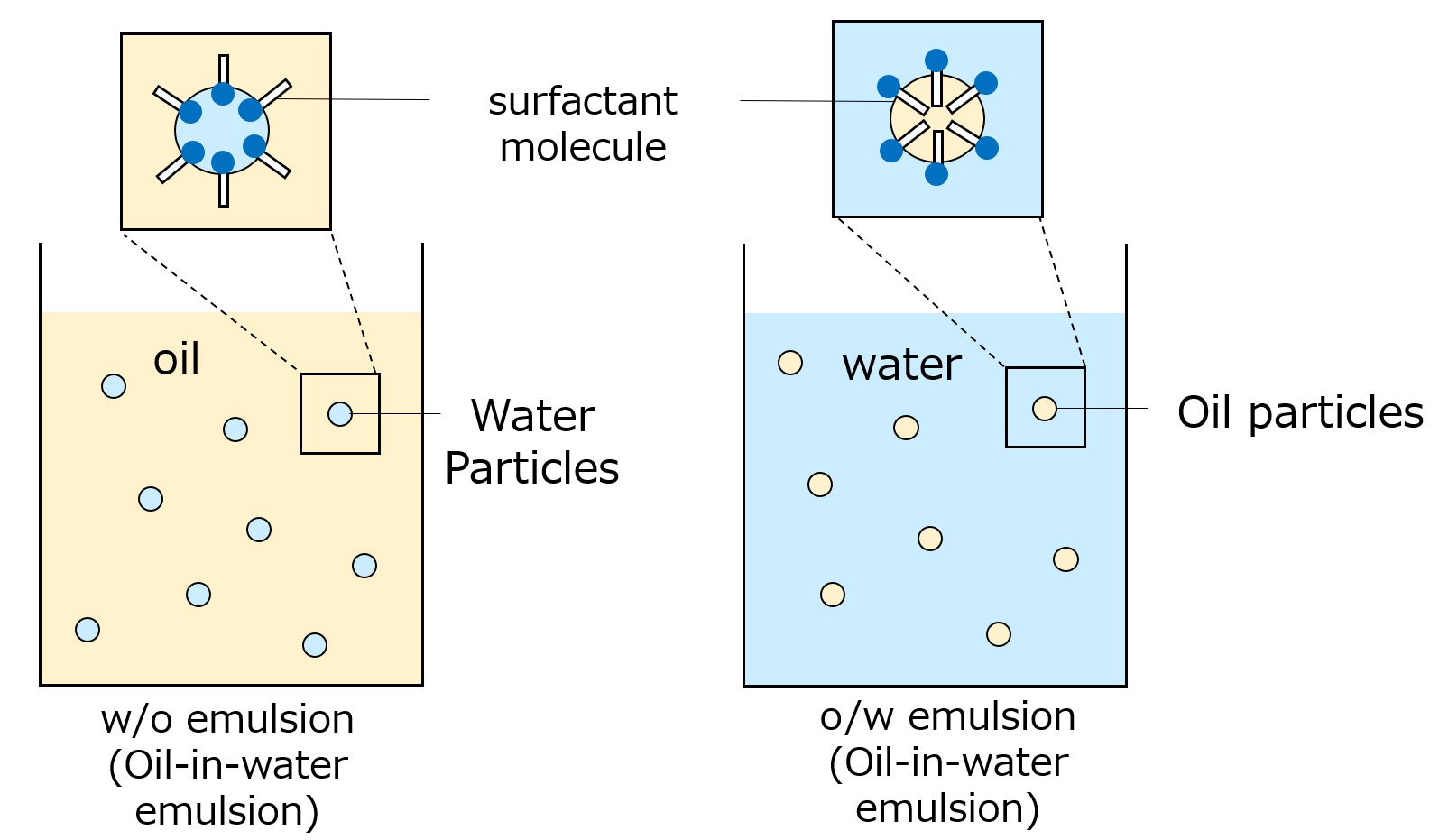
Fig. Conceptual diagram of w/o emulsion and o/w emulsion
Oil emulsified in water: o/w emulsion (oil in water emulsion)
Examples of natural products: milk, rubber tree sap
Water emulsified in oil: w/o emulsion (water-in-oil emulsion)
Example of natural product: crude oil
Examples of man-made emulsions
Cosmetic emulsion, cosmetic cream, herbicide, insecticide, car wax, floor wax, emulsion-type bath additive, water-based paint, water-based ink, water-based adhesive, textile oil, textile processing binder, textile finishing agent, paper processing binder, etc.
Emulsification and Solubilization Mechanisms
Why don't water and oil mix?
| Water | The molecule is polarized and electrically has a slightly positive charge and a slightly negative charge. |
|---|---|
| Oil | The molecules are not polarized and have little or no polarity. |
Surfactants work to mix water and oil
(1) Act to suppress repulsion at the interface between water and oil (lower surface tension)
When a surfactant is added to water and oil that do not mix as they are, the surfactant comes to the contact surfaces where the water and oil repel each other, orientating hydrophilic groups in the water and lipophilic groups in the oil and preventing the water and oil from repelling (lowering surface tension).
When a drop of water drips slowly, it tries to form a sphere after falling for a while.This is because the surface of the water has a contracting force called surface tension, which causes the droplet to take the smallest surface area shape.The table below shows examples of surface tension of liquids.
Example of surface tension of a liquid
| Liquid | Gas in contact with liquid | Temperature ℃ |
Surface tention mN/m |
|---|---|---|---|
| water | air | 20 | 72.8 |
| octane | octane vapor | 20 | 21.7 |
| benzene | air | 20 | 28.9 |
| Surfactant aqueous solution (sodium lauryl sulfate sodium salt 0.1vol%) |
air | 25 | 33.4 |
Compared to water, hydrocarbons such as octane and benzene have a lower surface tension.
Since the lipophilic groups of surfactants are mainly hydrocarbon groups, the presence of surfactants explains the decrease in surface tension at the water/air and water/oil interfaces, as shown in the figure below.
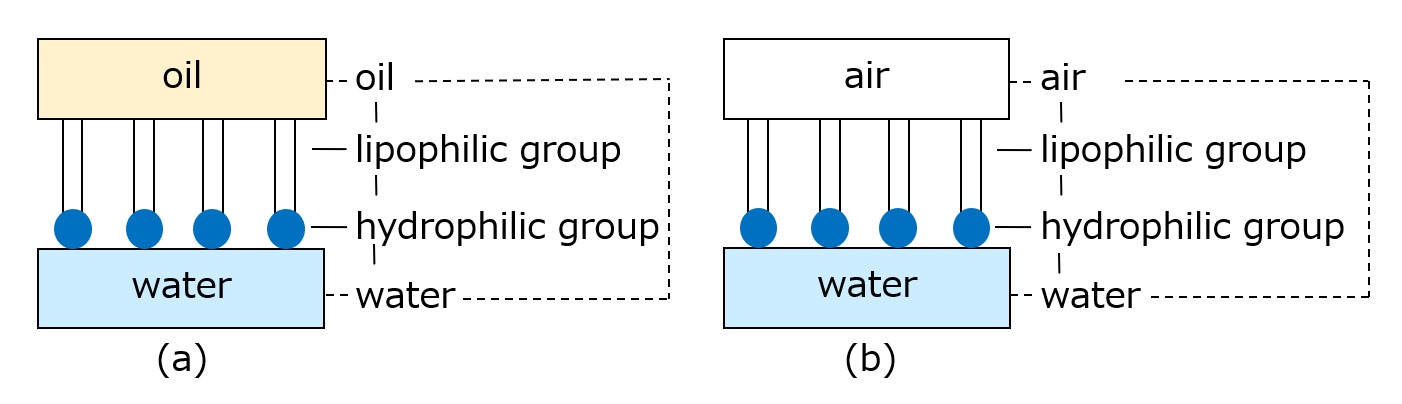
Fig. Conceptual diagram showing why surfactants reduce interfacial tension
In particular, in Figure (a) on the left, the surfactant eliminates the large repulsion between water and oil, and the interfacial tension (the tension when a liquid is in contact with another liquid or solid) drops to almost 0 mN/m.
(Oil and water, or air and water, do not need to be in direct contact due to the presence of surfactants.)
About critical micelle concentration (c.m.c.)
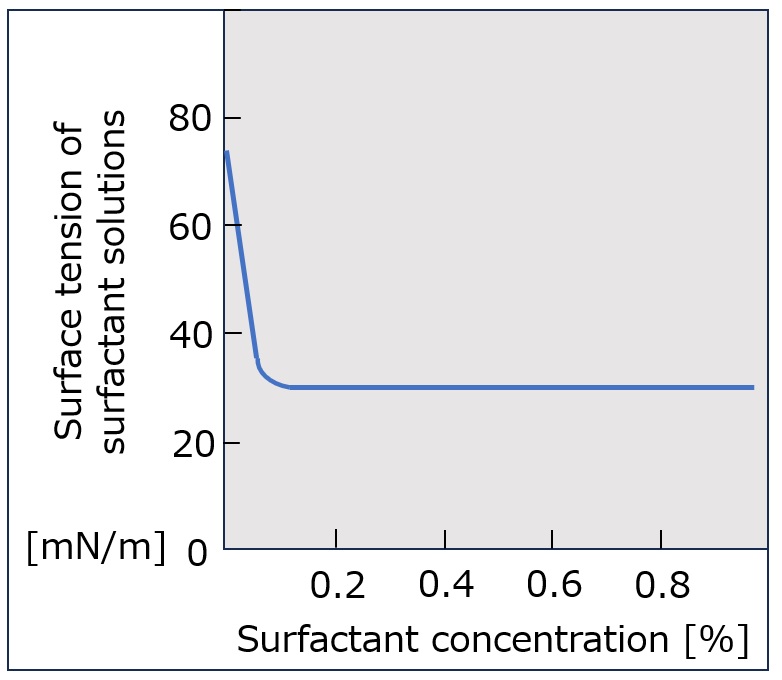
Fig. Relationship between concentration and surface tension of surfactant solutions
In relation to surface tension, an important property to know about surfactants is the critical micelle concentration (c.m.c.).
In aqueous solution, surfactants orient at the interface with air, which is dependent on the concentration. When the surface tension of an aqueous surfactant solution is measured while gradually increasing its concentration, the surface tension drops steeply at first and then becomes constant, as shown in the left figure, regardless of the type of surfactant.
A schematic drawing of this situation is shown in the figure below.
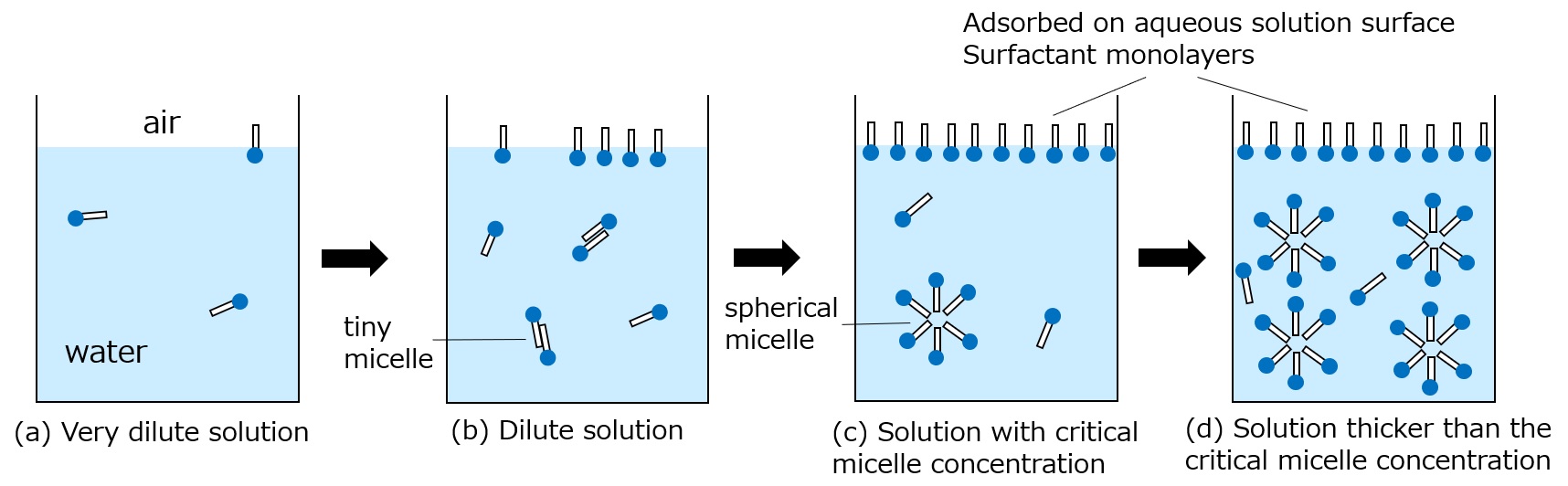
Fig. Relationship between surfactant behavior and changes in surfactant concentration
As the concentration of surfactant increases, it forms a monolayer tightly lined up on the surface of the aqueous solution, completely blocking direct contact between air and water (state (c)). This state corresponds to the point in the above figure where the curve of surface tension has dropped and is about to become horizontal, and from this concentration, at a slightly higher concentration, surfactant molecules gather in the aqueous solution by tens to hundreds, forming an aggregate (micelle) with their lipophilic groups facing inward and their hydrophilic groups facing outward. This surfactant micelle is called a "surfactant micelle. The lowest concentration at which these surfactant micelles form is called the critical micelle concentration (c.m.c.).
Relationship between critical micelle concentration (c.m.c.) and surfactants and their various effects
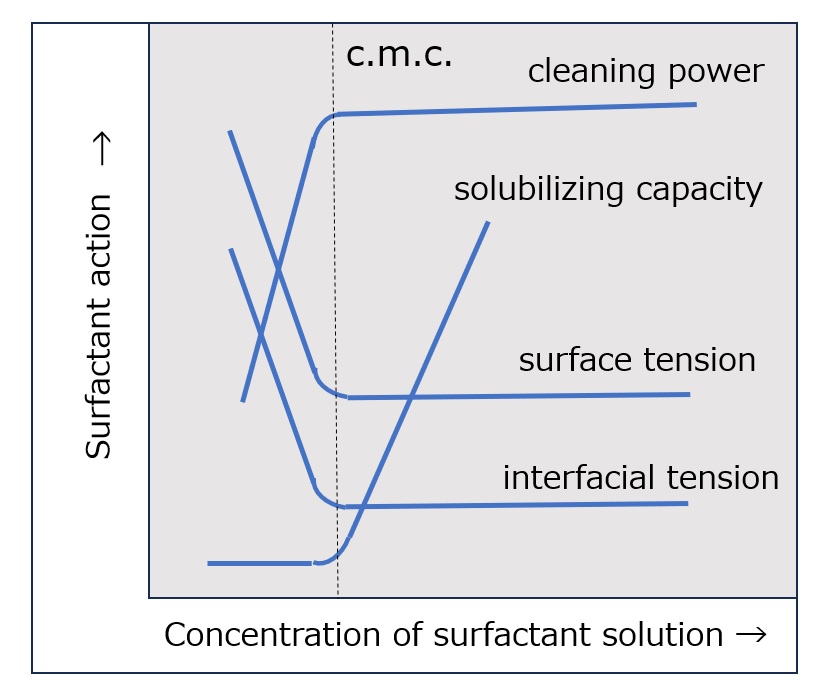
Fig. Relationship between critical micelle concentration and various surfactant actions
In aqueous solutions of surfactants, many physical properties such as electrical conductivity, osmotic pressure, freezing point depression, vapor pressure, viscosity, density, solubilizing capacity, and detergency change drastically around the critical micelle concentration (c.m.c.).
The relationship between critical micelle concentration (c.m.c.) and surfactant properties is shown in the figure on the left, which clearly shows how important it is to use surfactants at concentrations above the critical micelle concentration (c.m.c.).
(2) Electrical repulsive force (formation of electrical double layer)
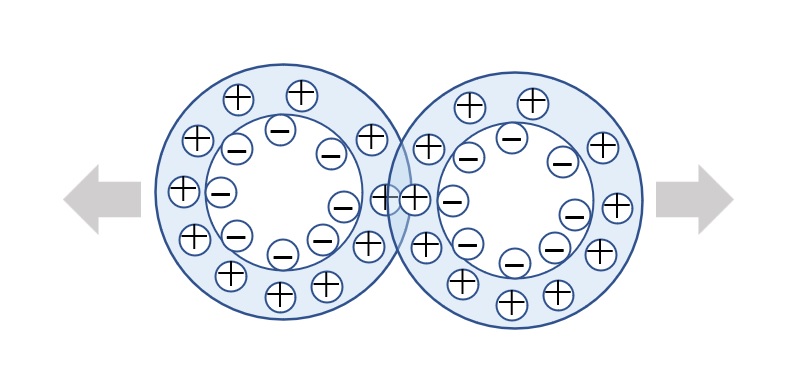
Fig. Conceptual diagram of electric double layer
When an emulsifier is used to emulsify oil in water, the emulsifier adsorbs on the surface of the oil with its hydrophilic groups facing the water phase. If the emulsifier used is an ionic surfactant in particular, counterions with opposite charges to the emulsifier's hydrophilic groups will collect near the emulsifier adsorbed on the oil.
These counterions are subject to Coulomb force, which is pulled by the charge on the surface of the particle, and thermal motion, which tries to cancel it out. The area where the Coulomb force is strong and the counterions are fixed to the particle surface is called the fixed layer, and the area where the thermal motion is strong and the counterions are not fixed is called the diffuse layer, which together is called the electric double layer.
The potential at the boundary between these two layers is called the zeta potential, and the larger this potential is, the stronger the repulsive force becomes, which prevents particles from colliding with each other and agglomerating.
(3) Three-dimensional repulsion (three-dimensional protective action)
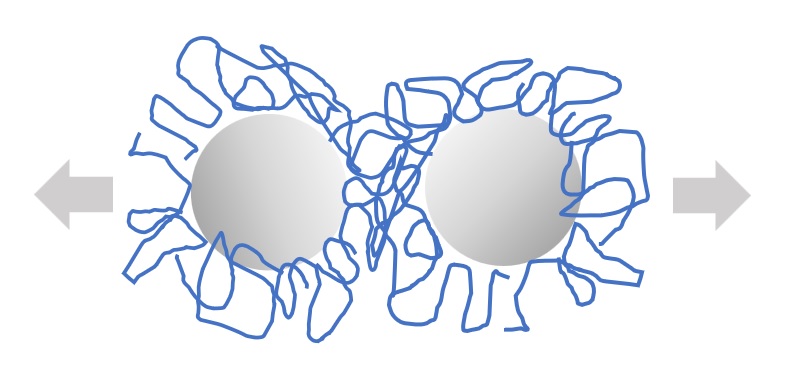
Fig. Schematic of stereoprotection action of polymer-type surfactants
In particular, the polymer type surfactant is characterized by steric repulsion due to its steric protective action.
The more strongly the surfactant is adsorbed on the oil particle surface or the thicker the adsorbed hydration layer, the stronger the steric repulsive action between particles and the less likely the oil particles are to aggregate. This steric protective action is greater the higher the molecular weight of the surfactant and the stronger the interaction of the surfactant's lipophilic groups with the oil particles.
Indicators for selecting emulsifiers (surfactants)
HLB(Hydrophile-Lipophile Balance)
In order to stably emulsify oils of different properties, such as vegetable oils and agricultural oils, into water, it is necessary to select an appropriate surfactant for each application.
This selection is strongly related to the balance between hydrophilic and lipophilic properties of surfactants, and HLB (Hydrophile-Lipophile Balance) has been proposed as an indicator to show this balance. The method of calculating HLB using the organic and inorganic properties of the surfactant as indices is convenient.
In this formula, the organic and inorganic properties of an organic compound are calculated from the values shown in the table below.
Organic and inorganic values
| Inorganic groups | Inorganic values |
|---|---|
| Light metals (salt) | More than 500 |
| Heavy metals (salts), amines and NH, salts | More than 400 |
| ーAsO3H2、 >ASO2H | 300 |
| ーSO2-NHーCOー、ーN=NーNH2 | 260 |
| ーSO3H、ーNHーSO2ーNHー | 250 |
| ーCOーNHーCOーNHーCOー | 250 |
| ーCOーNHーCOーNHー | 240 |
| ーSO2ーNHー | 240 |
|
ーCSーNHー、ーCOーNHーCOー |
230 |
| =NーOH、ーNHーCOーNHー | 220 |
| =NーNHー | 210 |
| ーCOーNHー | 200 |
| ーCOOH | 150 |
| lactone ring | 120 |
| ーCOーOーCOー | 110 |
| Anthracene ring, phenanthrene nucleus | 105 |
| ーOH | 100 |
| > Hg (covalent bond) | 95 |
| ーNHーNHー、ーOーCOーOー | 80 |
|
ーN< (ーNH2、ーNHR、ーNR2) aminic |
70 |
| >CO | 65 |
| ーCOOR,naphthalene nucleus(85), quinoline nucleus |
60 |
| >C=NH | 50 |
| ーN=Nー | 30 |
| ーOー | 20 |
| Benzene ring (general aromatic ring) | 15 |
| Ring (general aromatic monocycles) | 10 |
| Triple bond | 3 |
| Double bond | 2 |
| Organic and Inorganic groups | Organic values | Inorganic values |
|---|---|---|
| R4BiーOH | 80 | 250 |
| R4SbーOH | 60 | 250 |
| R4AsーOH | 40 | 250 |
| R4PーOH | 20 | 250 |
| >SO2 | 40 | 110 |
| ーCSSH | 100 | 80 |
| ーSCN | 90(70) | 80 |
| ーCSOH、ーCOSH | 80 | 80 |
| ーNCS | 90(70) | 75 |
| ーBi< | 80 | 70 |
| ーNO2 | 70 | 70 |
| ーSb< | 60 | 70 |
| ーAs<、 ーCN | 40 | 70 |
| ーP< | 20 | 70(20) |
| ーCSSR | 130 | 50 |
| ーCSOR、 ーCOSR | 80 | 50 |
| ーNO | 50 | 50 |
| ーOーNO2 | 60 | 40 |
| ーNC | 40 | 40 |
| ーSb=Sbー | 90 | 30 |
| ーAs=Asー | 60 | 30 |
| ーP=Pー、ーNCO | 30 | 30 |
| ーOーNO、ーSH、ーSー | 40 | 20 |
| ーI | 80(60) | 10(20) |
| ーBr | 60(40) | 10(20) |
| =S | 50 | 10 |
| ーCl | 40(20) | 10(20) |
| ーF | 5 | 5 |
| ーiso branching | -10 | 0 |
| tert branching ー>ー | -20 | 0 |
Organic values: determined as 20 per carbon atom
Inorganic value: According to the value in the table above. However
(1) When used in the calculation of HLB, oxyethylene (-CH2CH2O-) is treated specially and given an inorganic value of 75 and an organic value of 40.
(2) The organic property should be added separately for carbon atoms in the inorganic group.
(3) Carbon atoms in both organic and inorganic groups are added to the organic value.
(4) Although two literature values exist, the value in parentheses () is more appropriate for emulsification in many cases.
Solubility Parameter:SP value
Next, let us consider in detail why water and oil do not dissolve. The solubility parameter (SP value) is an important factor in determining whether water and oil dissolve into each other or are separated without dissolving.
This SP value is expressed as the square root of the aggregation energy density of the functional groups that make up the compound. Low molecular weight compounds can be compatible with each other even if these SP values are quite far apart, but high molecular weight compounds will not be compatible with each other if the SP values are usually about 1 or more apart.
Example:
Compound with high SP value: Water (SP value = 23.4)
Compound with low SP value: Toluene (SP value = 8.9)
Water and toluene do not dissolve each other.
Ionicity of emulsions and precautions when mixing
Compounds such as water and toluene, which do not dissolve into each other, can be mixed in an emulsion because each compound is emulsified in water.
Note that not all emulsions can mix with each other, so a little caution is required.The ionic properties of emulsion particles are an important controlling factor in whether emulsions can mix.Ionicity of emulsion particles refers to the electrical properties of the particles, which can be divided into anionic, cationic, and nonionic properties.
| Anionic emulsions | Surfactants with anionic groups (sulfonates, phosphates, carboxylates) bonded to lipophilic groups |
|---|---|
| Cationic emulsions | Surfactants with cationic groups (amine salts, quaternary ammonium salts) bonded to lipophilic groups |
| Nonionic emulsions | Surfactants with hydroxyl groups or polyethylene glycol chains bonded to lipophilic groups |
When these emulsions, anionic and cationic emulsions, are mixed, the particles stick to each other and agglomerate, destroying the emulsion (if they are the same ion or a combination of nonionic emulsions, the emulsion can usually be mixed without destroying the emulsion). (If the same ions are mixed with each other or non-ionic emulsions are combined, the emulsion is usually not destroyed.)
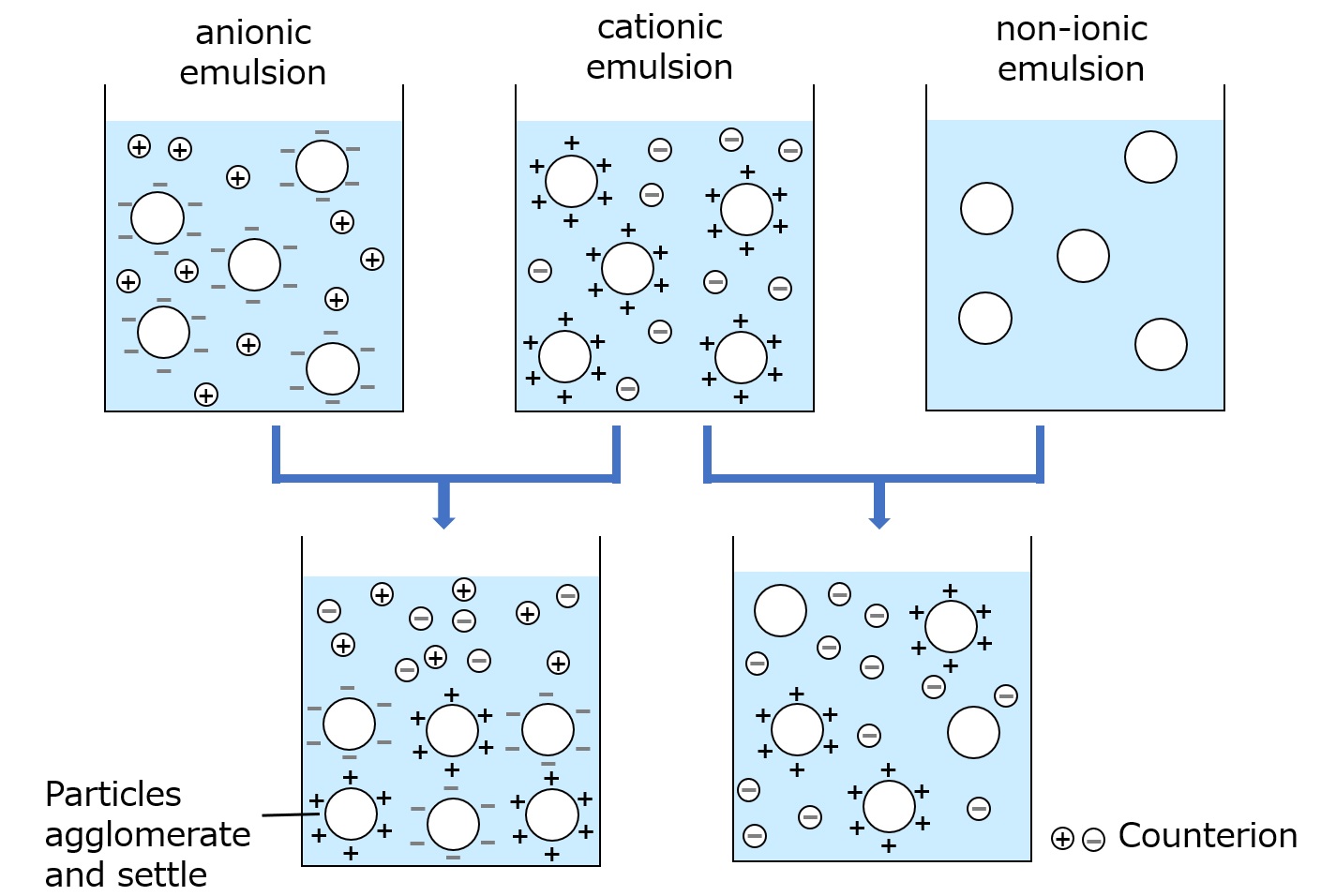
Fig. Change of state when emulsions with different ionic properties are mixed
Emulsifier Applications
Role of Emulsification in Coatings and Adhesives
In the field of paints and adhesives, solvent-based and emulsion-based systems are used separately, depending on durability and the nature of the adherend.
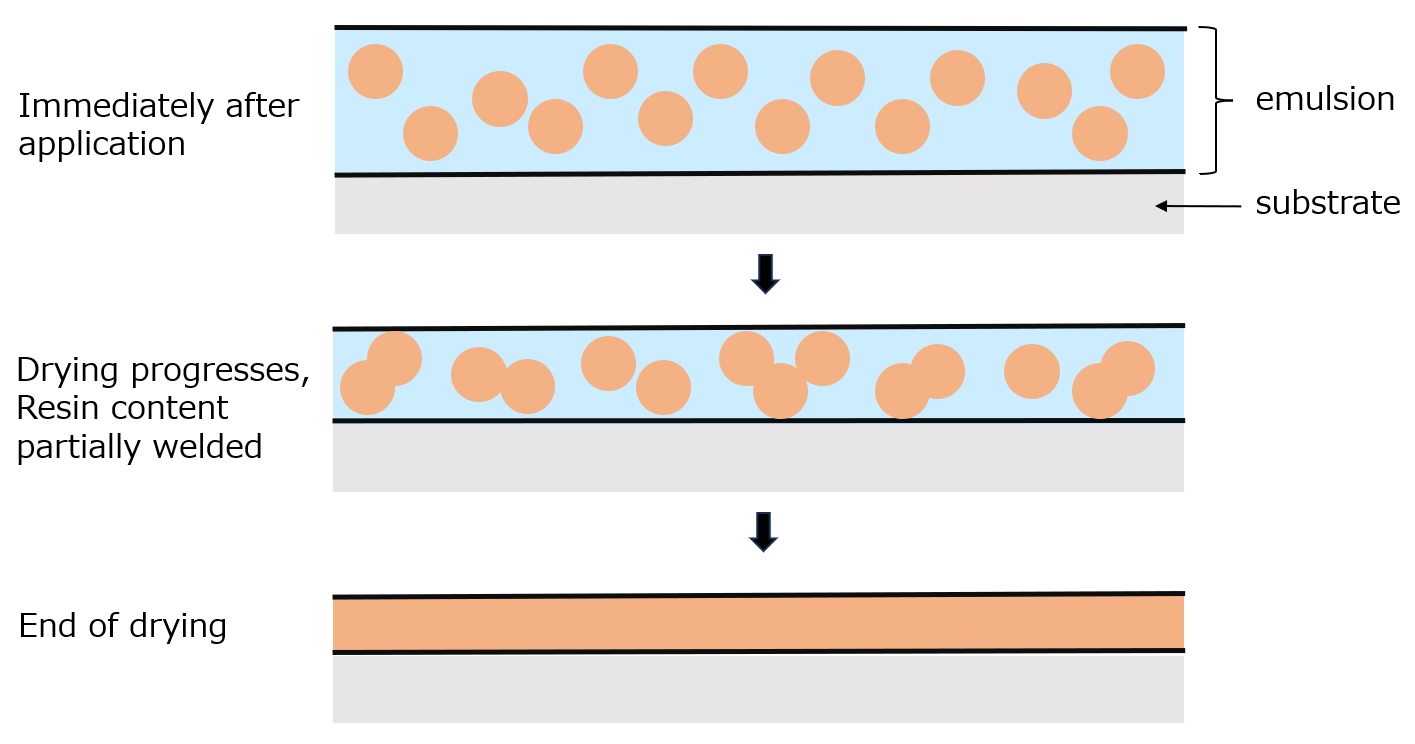
Fig. Drying process of emulsion paint
Emulsion-based paints and adhesives are fine emulsions of water-insoluble resins, but once they dry and harden, they do not emulsify again even when wet.
As shown in the figure on the left, this is due to the fact that the finely emulsified resin in the emulsion state increases in density as the water evaporates, causing the resin particles to fuse with each other and form a uniform film.
Role of emulsifiers for emulsion polymerization
Water-based paints and water-based adhesives are mainly composed of synthetic resin emulsions, and most of these synthetic resin emulsions are synthesized by a method called emulsion polymerization.
Emulsion polymerization is a method in which monomers with unsaturated double bonds are polymerized in micelles of an emulsifier to achieve high molecular weight. The molecular weight of the resulting resin usually exceeds 100,000, making it a very efficient method for obtaining high molecular weight resins while emulsifying them in water.
About Reactive Emulsifiers
Emulsions with reactive emulsifiers, which combine unsaturated double bonds and emulsification in a single molecule, have also been developed. In emulsions using non-reactive emulsifiers, the emulsifier tends to be released from the resin when it dries to form a film, causing a decrease in adhesion to the adherend. On the other hand, this reactive emulsifier chemically bonds with the resin and is incorporated into the resin, so it is not released from the resin when the emulsion dries to form a film, resulting in excellent adhesive performance.
Textile oils
Clothing is made from natural fibers such as cotton, wool, and silk, and synthetic fibers such as polyester and nylon. These synthetic fibers are manufactured by synthesizing polymers through a polymerization reaction of monomers, which are then stretched into thin strips with diameters ranging from 1 to 100 μm. Synthetic fibers are strong and can be freely modified in terms of thickness and shape to provide a variety of functions, and today their production volume exceeds that of natural fibers.
The following is a list of textile oils used in the production of synthetic fibers.
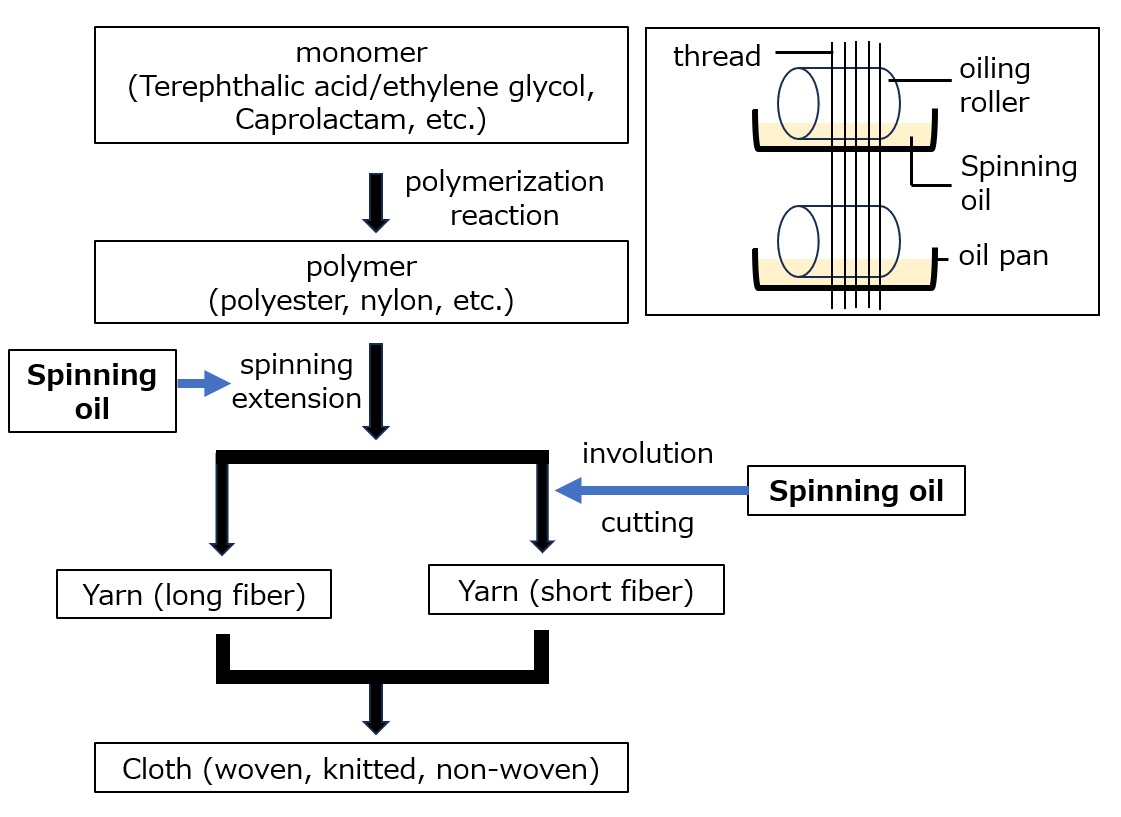
Fig. Synthetic fiber manufacturing process and oils for textiles
As shown in the figure below, polyester and nylon fibers are manufactured by extracting the polymer in a molten state, obtained by reaction at high temperatures, through narrow holes and stretching it at high speed.
In this yarn-making stage, fiber oils (e.g., ester or polyether synthetic lubricants, phosphate ester or aliphatic alkanolamide antistatic blends) with the following functions 1) to 3) are used.
(1) Improvement of smoothness
(Slides well when the yarn comes in contact with metal rolls, etc.)
(2) Antistatic properties
(When fibers rub against each other, they do not become charged with static electricity.)
(3) Focusing property
(Keeps yarns made by collecting single fibers together for easy handling in the processing process from falling apart.)
None of these functions are necessary in the final product, and since such oils sticking to the yarn cause uneven dyeing when coloring the cloth, the textile oils are usually removed in the refining process prior to dyeing. Therefore, although the textile oil does not remain in the final product, it plays an extremely important role in the efficient production of synthetic fibers.
Now, this oil for textile is often given to yarns in the form of oil emulsified in water, or oil-in-water emulsion, mainly because of the following reasons.
(1) Components with excellent smoothness are strongly hydrophobic and usually do not dissolve in water, so in order to uniformly adhere the minimum amount required, they are made into an emulsion so that they can be freely diluted with water.
(2) Water as a dispersant has a large heat capacity and latent heat of evaporation, making it excellent for cooling yarns. In addition, water as a diluent is optimal because it is inexpensive and free from fire and other hazards.
Textile Finishing Agents
Chemicals that impart various functions to synthetic fibers after dyeing are called textile finishing agents, which are also used in the form of emulsions.
Examples of functions required for fibers include flexibility, texture such as resilience and elasticity, water and oil repellency, water absorbency, sewability, antistatic properties, antibacterial properties, wind resistance, anti-pilling properties, and various others.
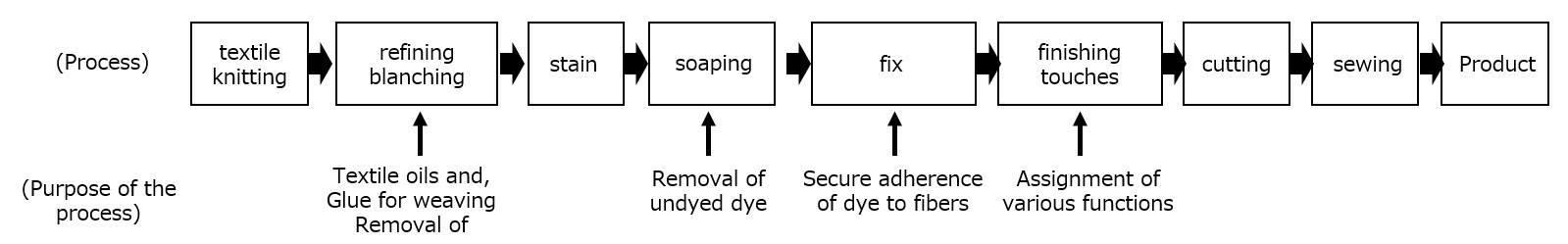
Fig. How textile products are made
As mentioned above, textile finishing agents are agents that provide a great many functions, and no single agent alone can meet these requirements. Therefore, several types of agents must be mixed together, and this is where the emulsification function plays a major role.
In order to make a homogeneous solution, whether it is an aqueous solution or an organic solvent solution, the solute (what is dissolved in the solvent) must be dissolved in the solvent; it is easy to dissolve one solute in one solvent, but often impossible to dissolve several solutes with different properties in the same solvent.
| Solute | Polyvinyl alcohol | Wax | Polyvinyl alcohol +wax |
|---|---|---|---|
| When mixed with water | soluble | insoluble | melting residue |
| When mixed with toluene | insoluble | soluble | melting residue |
The table above shows examples of wax and polyvinyl alcohol as solutes and their dissolution in solvents.
Wax has low polarity, so it dissolves in toluene, a non-polar solvent, but not in water, a polar solvent. On the other hand, polyvinyl alcohol has high polarity and is soluble in water but not in toluene. Thus, the properties of the solvent and the solute, i.e., the SP values, must be close to each other to ensure uniform dissolution.
Therefore, these solutes with different properties (in this case, various finishing agents) are converted into one liquid by emulsification.
| Functional Classification | Main composition | SP value |
|---|---|---|
| Fabric softener | higher alkyl quaternary ammonium salts | 9 |
| Water and oil repellents | silicone resin | 8 |
| fluoropolymer | 6 | |
| Water absorbent | special polyether type surfactant | 10 |
| Sewability improver | polyethylene wax | 9 |
| Antistatic agent | phosphate esters, etc. | ≧10 |
| Texture modifier (resilience) | polyurethane resin | 10~11 |
| Texture conditioner (hard finishing agent) | polyvinyl alcohol | 19 |
Agrochemical Field
Some pesticides are called emulsions. This is a liquid form of pesticide to which an emulsifier or other agent has been added, and is designed so that the pesticide can be easily emulsified into water by pouring the emulsion into the water. While most emulsions are produced by mechanically applying shear force, pesticides are designed to be naturally emulsified (emulsified by simply adding oil to water) because they are used by farmers who do not have such machinery and by ordinary households.
Contribution of emulsifiers
(1) Lowering the interfacial tension between water and oil
(2) Giving an electric charge to the particle surface and repulsion between particles
(3) Forming an adsorption film on oil particles to protect the particle surface
As an emulsifier, a surfactant having both lipophilic and hydrophilic groups in a single molecule is usually used, so it has the action of (1). In addition, by using ionic surfactants (in combination), the action (2) can be added. In selecting the best emulsifier to emulsify a certain oil, the most difficult part is usually the optimization of the effect of (3). In order to efficiently achieve the action of (3), it is important that the emulsifier has strong interaction with oil particles and good affinity with water (balance of lipophilic and hydrophilic groups).
Cosmetics
Cosmetics are broadly classified into skin care cosmetics, skin cleansing cosmetics, and finishing (makeup) cosmetics, This page introduces skin care cosmetics, especially cosmetic emulsions and creams, which are closely related to the emulsifying function.
The role of cosmetic emulsions and creams is to moisturize and soften the skin, especially the stratum corneum, the outermost layer of the skin, by replenishing moisture and oil.Emulsions, which emulsify oil in water, are therefore convenient because they provide moisture and oil to the skin at once and in relatively flexible proportions. This is where the emulsifying function plays an important role.
Related Prodcts and Topics(Surfactants, Emulsifiers)
As a surfactant manufacturer, we offer a lineup of numerous emulsification and solubilization-related products.
Sanyo Chemical's emulsifiers for emulsion polymerization
The structure and features of our emulsifiers are shown in the table below. Nonionic and anionic emulsifiers are available, and each has its own characteristics in terms of emulsification, dispersion stability, particle size, etc. They are selected according to the purpose, and are often used in combination.
| Reactivity | Ionicity | Product name | Structure | Feature |
|---|---|---|---|---|
| Non-reactive | Nonionic | EMULMIN products | polyoxyethylene alkyl ether | Good biodegradability |
| NAROACTY CL products | polyoxyalkylene alkyl ether | Equivalent performance to alkyl phenyl ether systems | ||
| Anionic | ELEMINOL CLS-20 |
Polyoxyalkylene alkyl ether sulfate ammonium | Equivalent performance to alkyl phenyl ether systems | |
| SANDET ONA |
Sodium 2-ethylhexyl sulfate | Suitable for emulsion polymerization of emulsions with large particle size | ||
| SANMORIN OT-70 |
Sodium dioctyl sulfosuccinate | Provides penetration and leveling properties | ||
| Reactive | Anionic | ELEMINOL JS-20 |
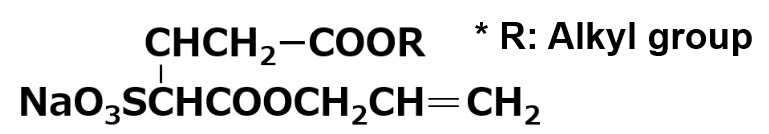 |
Excellent copolymerization with acrylic monomers. Emulsions with excellent mechanical and chemical stability can be obtained. |
Related products (surfactants, emulsifiers and solubilizers)

- Nonionic surfactant
Nonionic Surfactants Catalog
Sanyo Chemical's main lineup of nonionic surfactants

- Nonionic surfactant
Polyoxyethylene - Polyoxypropylene Block Copolymer ”NEWPOL PE”
Lineup of products with distinctive features and a wide variety of functions can be added

- Nonionic surfactant
Polyoxyalkylene lauryl ether-type nonionic surfactant"EMULMIN FL, HL"
"EMULMIN FL and HL" products are non-ionic surfactants (polyoxyalkylene lauryl ether) made from natural lauryl alcohol.

- Polyethylene glycol
Polyethylene glycol"PEG"
PEG is highly water soluble and is not hydrolyzed, so it is widely used as a water soluble base for viscosity adjustment and other applications.

- Anionic surfactant
Sulfosuccinate-type anionic surfactant ”SANMORIN OT-70”
Approximately a 70 mass% solution of a sodium dioctyl sulfosuccinate that has particularly excellent penetrating power and surface tension lowering ability among surfactants.
Related topics


This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.





