Surfactant Basics 4 (Dispersants)
- What is a surfactant
- Surfactant functions introduction video
- What is a dispersant
- Paints and Dispersants
- Printing Inks and Dispersants
- Paper and Dispersants
- Cosmetics and Dispersants
- Cement and dispersants
- Pesticides and dispersants
- Dyeing and Dispersion
- Plastic coloring and dispersion
- Related Information
What is a surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
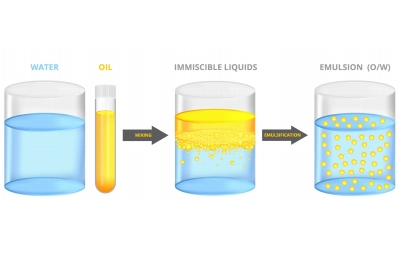
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
Basic structure of a surfactant
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-fitting parts) and hydrophilic groups (water-fitting parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant |
-Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH |
-Clothing detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil |
-Polyoxyethylene alkyl ether etc. |
| Anionic surfactant |
-Excellent emulsification and dispersibility -Good lather -Temperature insensitive |
-Clothing Detergent -Shampoo -Body soap |
-Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant |
-Adsorption to fibers -Antistatic effect -Bactericidal |
-Hair rinse -Fabric softener for clothes -Disinfectant |
-Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant |
-Mild on skin -Excellent solubility in water -Synergistic with other active components |
-Shampoo -Body soap -Kitchen detergent |
-Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant functions introduction video
What is a dispersant?
How dispersants work
Agents that perform dispersing functions are called dispersing agents. For example, they break up solid particles one by one and disperse them uniformly in a dispersing medium, and they also prevent the dispersed particles from re-agglomerating and maintaining a stable dispersed state.
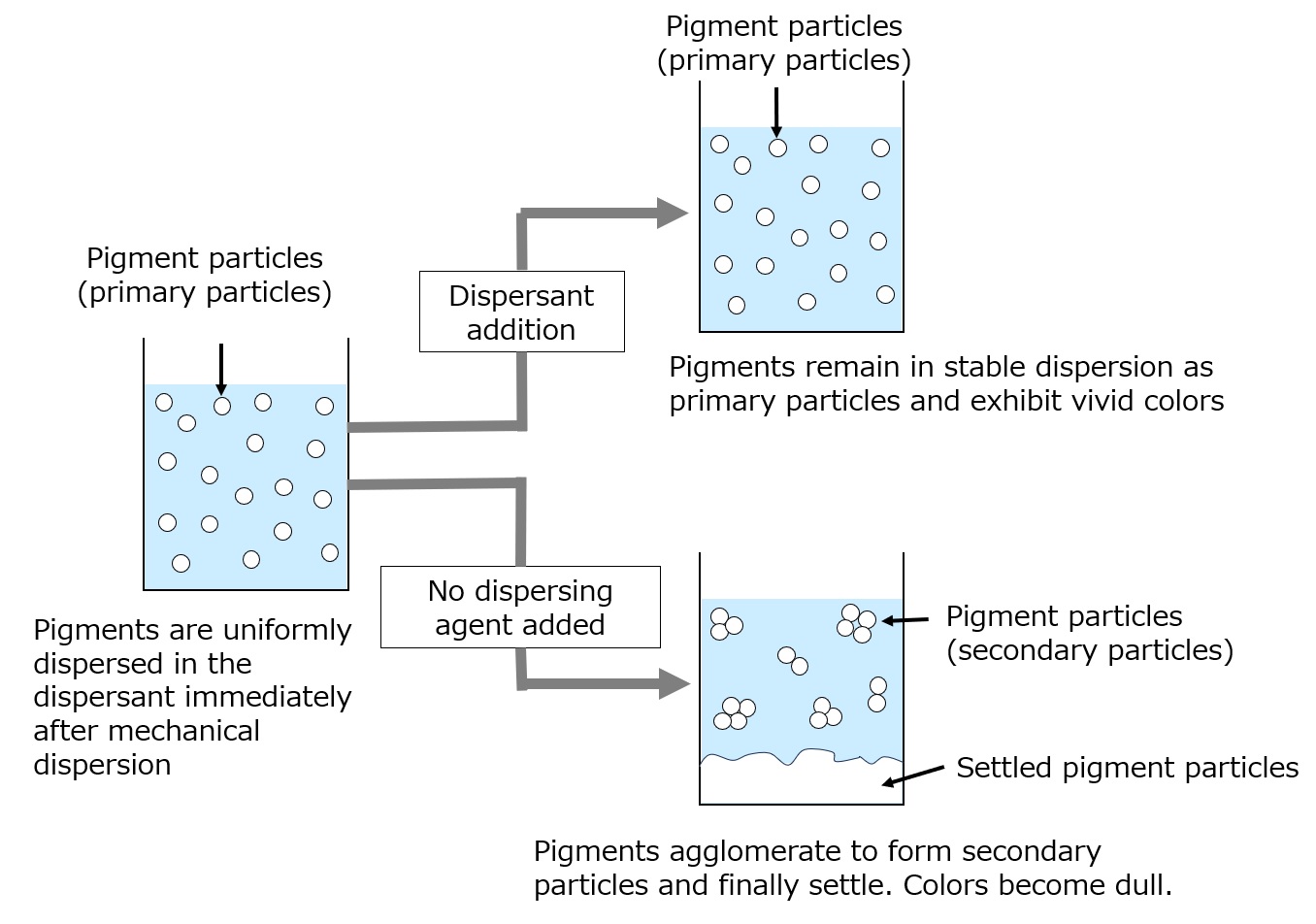
Fig. How dispersants work
Adsorption and dispersion stabilizing action of dispersants on particles
Dispersants have a chemical structure that has an affinity for both solid particles and liquids, and have functional groups that adsorb to the particle surfaces.
This adsorption results in the particle surfaces being covered with an adsorbed layer of charged dispersant, increasing the electrostatic repulsive force between particles and stabilizing their dispersion.
For example, as shown in the figure below, when a surfactant with an ionic group of opposite sign to the charge of a particle (A) dispersed in water is added, the particle becomes hydrophobic (lipophilic) because the hydrophilic part faces the particle and the opposite side becomes lipophilic, forming an adsorption layer as shown in (B). When various surfactants are added, adsorption occurs with the lipophilic part on the adsorption layer and the hydrophilic group on the outside, as shown in (C), (D), and (E), and the entire particle becomes hydrophilic, making it easier to wet in water and at the same time increasing electrostatic repulsion and stabilizing dispersion.
In the case of polymer-type dispersants, in addition to this electrostatic repulsion, the repulsive force due to steric hindrance of the polymer chain is added, which further improves dispersibility.
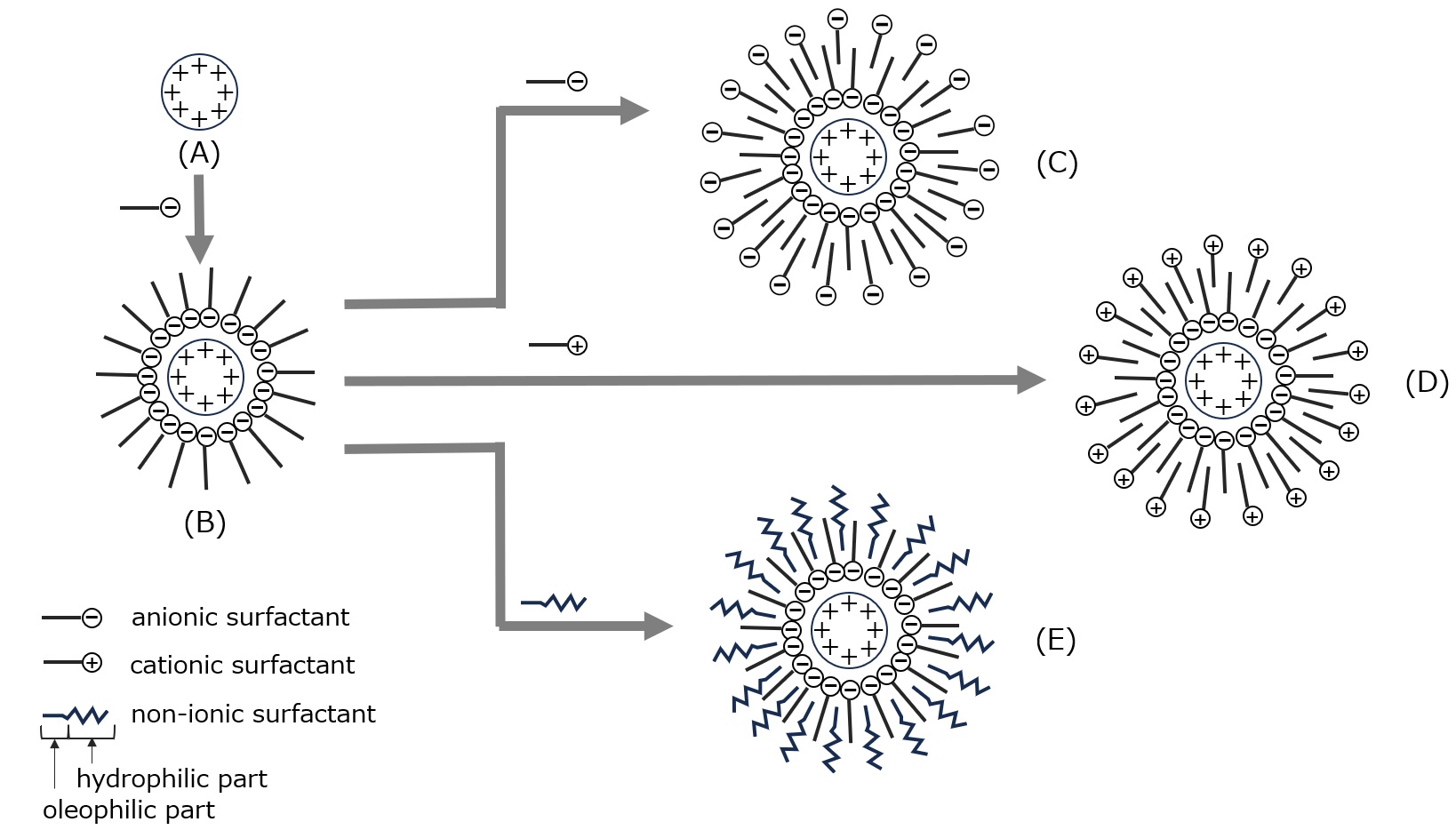
Fig. Adsorption of surfactant on charged particles
Dispersant Classification
Dispersants can be divided into three types: (1) polymer-type dispersants, (2) surfactant-type dispersants, and (3) inorganic-type dispersants (such as polyphosphoric acid).
The characteristics of each dispersant are summarized in the table below.
Dispersant Types and Characteristics
| Dispersant type | feature |
|---|---|
| Polymer-type dispersants (e.g., polycarboxylic acid polymers) |
In addition to electrostatic repulsive force, there is a repulsive effect due to steric hindrance of polymer chains (protective colloid action). Dispersions with good long-term stability can be obtained. |
| Surfactant-type dispersants (nonionic, anionic surfactants, etc.) |
Adsorbs on the pigment surface and lowers the interfacial energy, making the particle surface more wettable in water and organic solvents. Excellent wetting effect. Although electrostatic repulsion has a dispersing effect, it is weak, so other dispersing agents are often used in combination. |
| Inorganic dispersants (e.g. polyphosphate) |
Excellent dispersion in aqueous systems. Dispersibility deteriorates over time due to susceptibility to hydrolysis. |
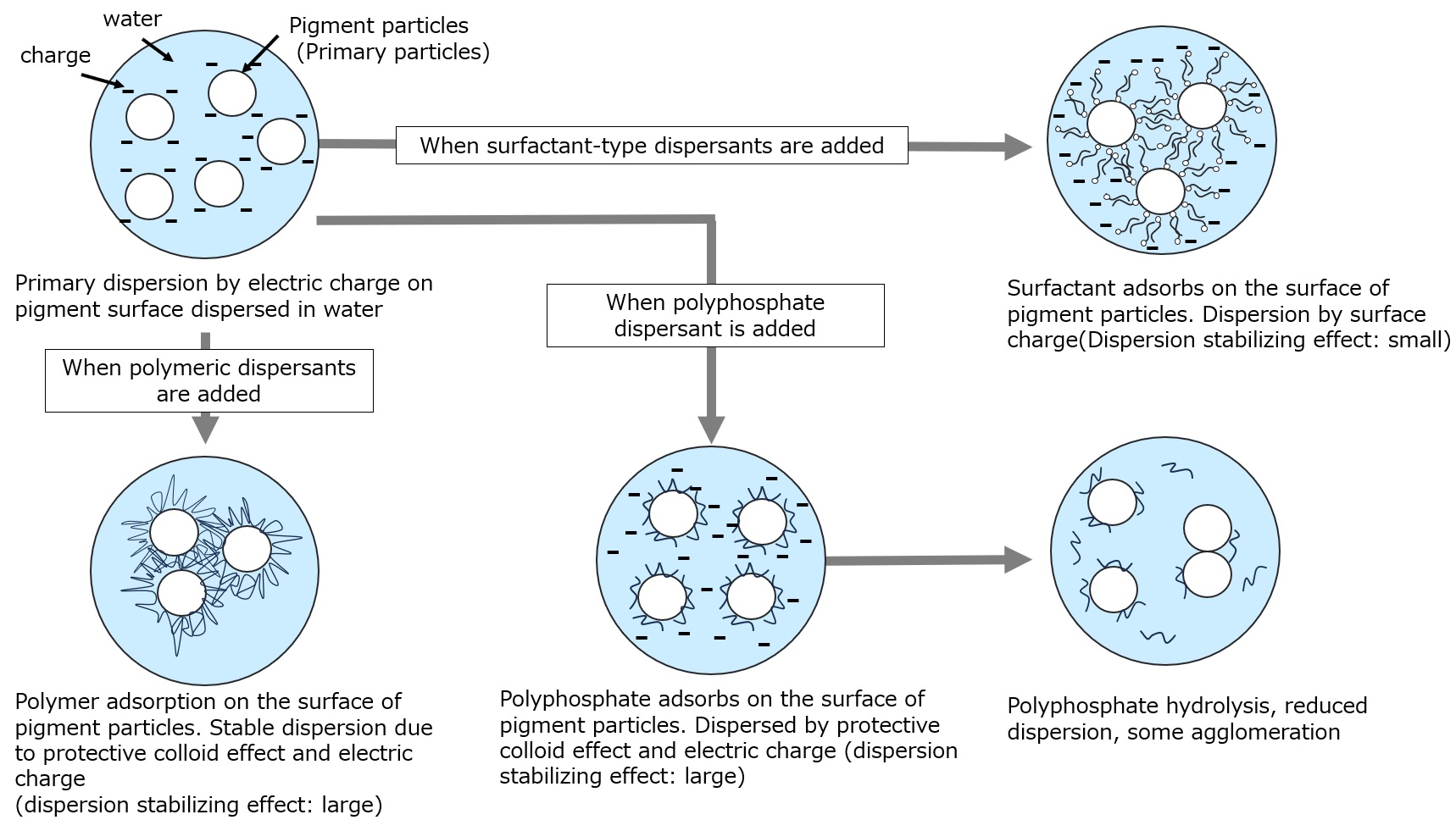
Fig. Dispersant Action Mechanism Model
Points to consider when selecting an aqueous dispersant
A wide variety of dispersants are used in various fields, depending on the nature of the particles to be dispersed (hydrophilic or hydrophobic surface) and the type of dispersant (dispersed in water or oil).
Important point when water is the dispersant
-Select a dispersant that is soluble in water and easily adsorbed on the particles to be dispersed.
-The finer the particle diameter, the more cohesive and difficult dispersion becomes, so it is better to use a wetting surfactant that reduces interfacial energy.
-When the particle concentration is high, a high molecular weight type is effective as it is expected to have repulsive force due to steric hindrance.
Sedimentation phenomena and particle settling velocity (Stokes' equation)
Sedimentation is a phenomenon that plays a major role in the stability of solid-liquid dispersion systems. The sedimentation velocity of particles, v, is discussed by the following equation called "Stokes' law. The following equation shows that the sedimentation velocity of particles decreases with decreasing particle size, decreasing density difference between particles and dispersant, and increasing viscosity of dispersant.
v=2r2 (ρーρp)g/9η
r: radius of particle,
ρ: density of particle,
ρp: density of dispersant,
η: viscosity of dispersant,
g: gravitational constant
Forces acting on dispersed particles (electrostatic repulsive forces, van der Waals forces)
Two forces generally act on particles dispersed in a liquid.
-Electrostatic repulsive force based on the charge on the particle surface
-Van der Waals force (cohesive force)
Whether particles disperse or agglomerate depends on the combination of these two forces. If the surface charge is high and the repulsive force is high, the dispersion is stable. On the other hand, if the cohesive force is high, the particles agglomerate, and the difference in specific gravity between the particles and the dispersant causes them to settle and separate.
Electric double layer (electrostatic repulsive force) and DLVO theory
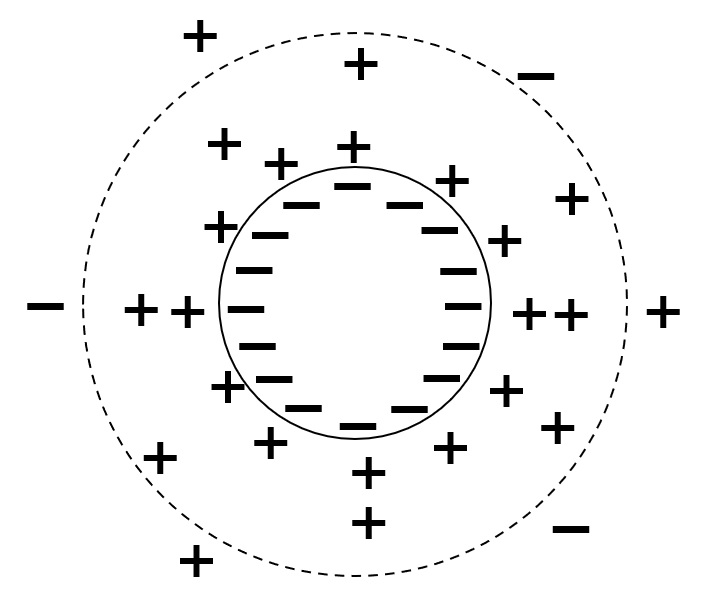
Fig. Conceptual diagram of electric double layer
Stability due to electrostatic repulsive forces is explained by the following electric double layer.
For example, when clay particles are suspended in water, as shown in the left figure, there is an electrical charge on the surface of the particle, which surrounds it and adsorbs ions of opposite charge on the outside. These ions are distributed in a certain spread from the particle surface in water, forming a diffuse electric double layer composed of negative and positive charges.
In a liquid, when particles approach each other, they first collide with their outer counterions, causing electrical repulsion. Many rivers are muddy because clay particles have a negative charge and form an electric double layer in the water, which prevents agglomeration between particles and keeps them stable without sinking.
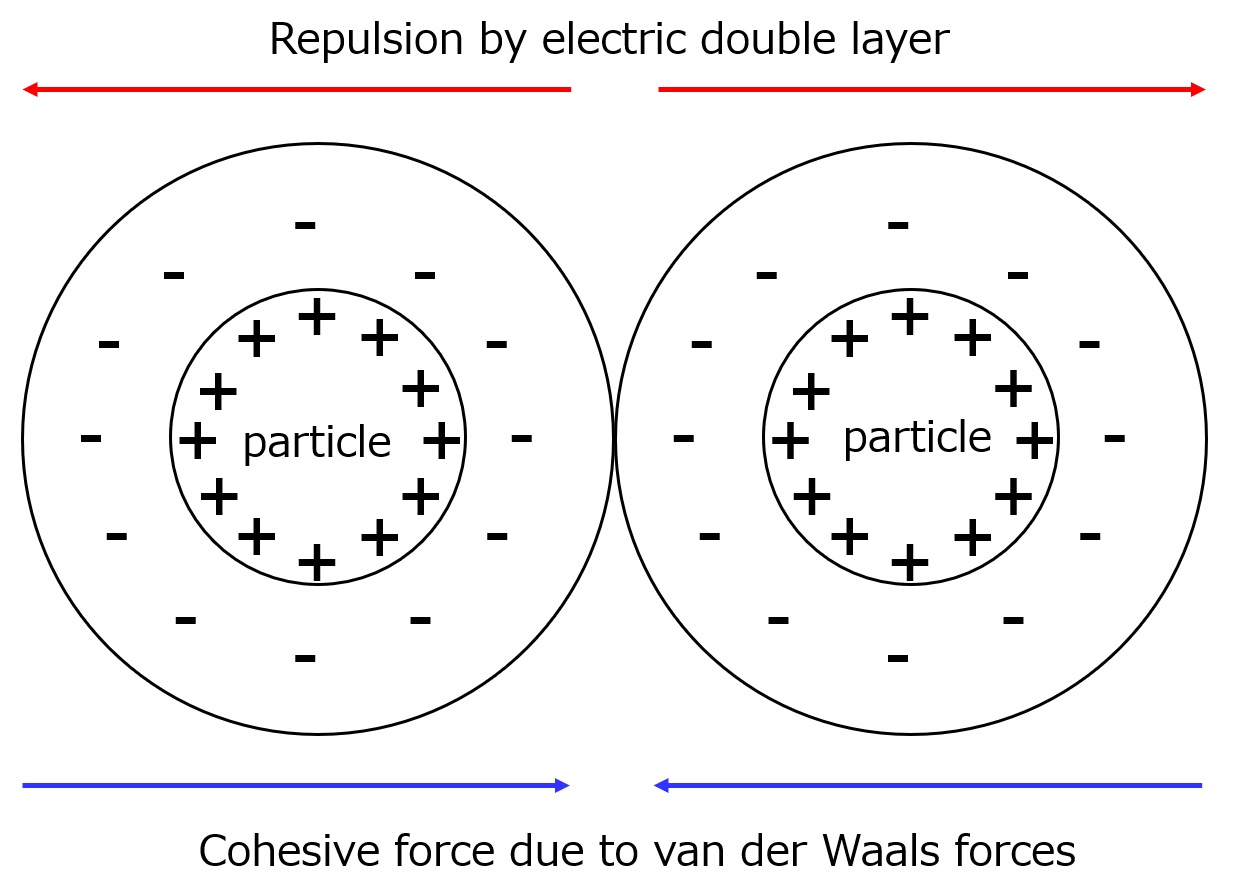
Fig. Schematic diagram of forces acting on particles
As shown in the left figure, when the electric double layer is thick, even if particles with the same type of double layer approach each other, the repulsive force between charges of the same sign in the double layer acts more than van der Waals forces, and agglomeration becomes difficult.
However, as the electric double layer becomes thinner, the van der Waals forces capture the approaching particles and they agglomerate.
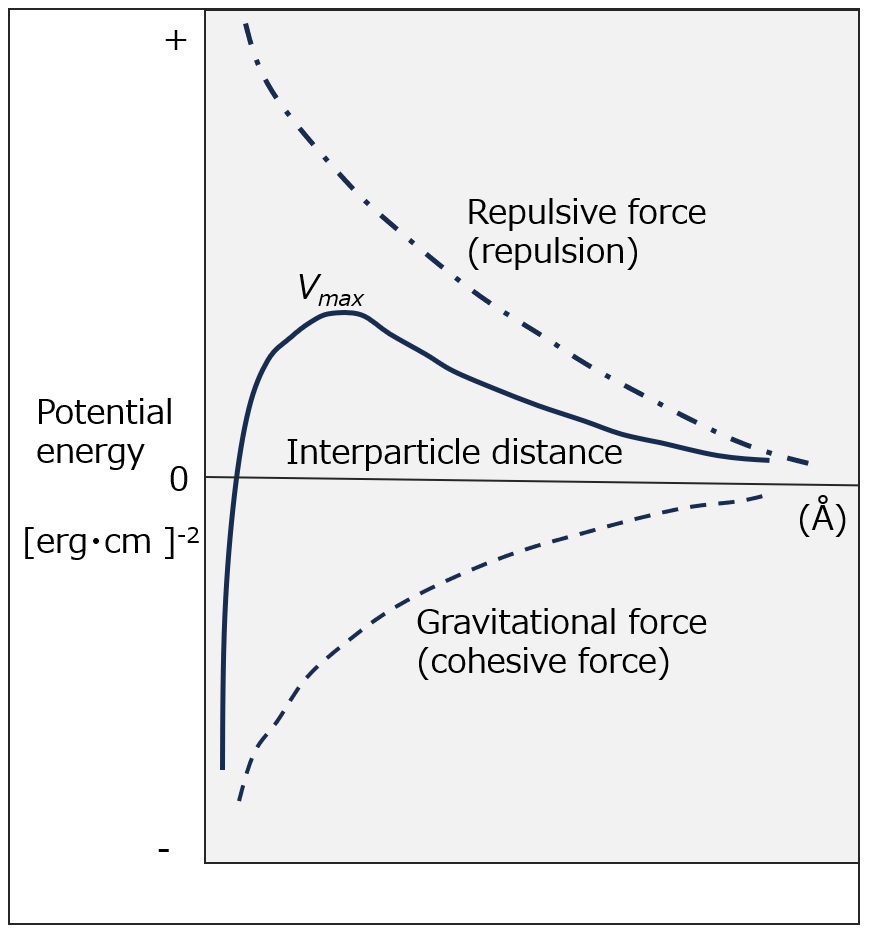
Fig. DLVO Theory
DLVO Theory
Whether a dispersive system is stable or not depends on how the combined repulsive and attractive forces vary with the distance between particles.
This is summarized in the "Theory of DLVO" (named after the initials of Derjaguin, Landau, Verwey, and Overbeek). The repulsive and attractive forces and their sums plotted against the distance between particles are shown in the figure on the left.
In this case, the higher the potential barrier (Vmax) formed by the sum of the repulsive and attractive force curves, the more stable the dispersive system is. v represents the total potential consisting of the electrostatic repulsive potential and the van der Waals attraction potential.
HLB of surfactant type dispersant
HLB (Hydrophile-Lipophile Balance) is a useful method for selecting dispersants.
This is a numerical value that expresses the degree of hydrophilicity/lipophilicity of a surfactant, and the affinity between the dispersant and the dispersed material is viewed from the balance of hydrophilicity and lipophilicity (hydrophobicity). In other words, if a dispersant is used that is close to the HLB of the dispersed material, dispersibility will be better in many cases.
An example of HLB is shown in the table below: the lower the HLB, the more lipophilic (hydrophobic) the dispersant is, and the higher the HLB, the more hydrophilic the dispersant is.
An example of HLB of surfactants
| Classification | surfactant | HLB |
|---|---|---|
| Higher alcohol EO adduct | Lauryl alcohol EO 5 mol adduct | 10.8 |
| Lauryl alcohol EO 10 mol adduct | 14.1 | |
| Lauryl alcohol EO 23 mol adduct | 16.9 | |
| Oleyl alcohol EO 2 mol adduct | 4.9 | |
| Oleyl alcohol EO 10 mol adduct | 12.4 | |
| Oleyl alcohol EO 20 mol adduct | 15.3 | |
| Polyalcohol ester | Monoglycerides (industrial use) | 2.8-3.5 |
| Sorbitan laurate monoester | 8.5 | |
| Sorbitan palmitate monoester | 6.7 | |
| Sorbitan stearic acid monoester | 4.7 | |
| Sorbitan oleic acid monoester | 4.3 | |
| Sorbitan oleate triester | 1.8 |
About Polymer Type Dispersants
Polymer-type dispersants are able to stably disperse pigments and other substances by the protective colloidal effect and electric charge of polymers adsorbed on the surface of pigment particles.
When selecting a polymeric dispersant, attention should be paid to its molecular weight in addition to its chemical structure.If the molecular weight exceeds several hundred thousand, agglomeration is likely to occur. Molecular weight distribution is also known to affect the dispersion effect.
Typical polymeric pigment dispersants
| Type | Compound name | Example of chemical structure | Feature / Other |
|---|---|---|---|
| Aqueous dispersants | Naphthalene sulfonate | 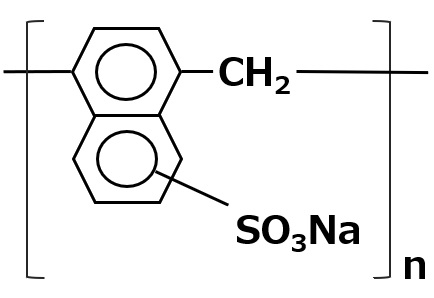 |
-Organic and inorganic pigments, etc. -Effective for a wide range of pigment dispersion -Not affected by water hardness or pH |
| Aqueous dispersants | Polystyrene sulfonate | 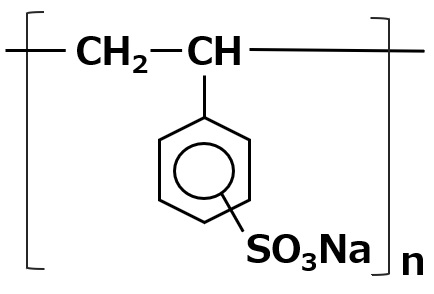 |
-Effective for dispersion of hydrophobic pigments -Not affected by water hardness or pH |
| Aqueous dispersants | Polyacrylate |
|
-Effective for dispersion of hydrophilic inorganic pigments |
| Aqueous dispersants | Vinyl Compounds and Carboxylic acid monomer Salts of copolymers of | 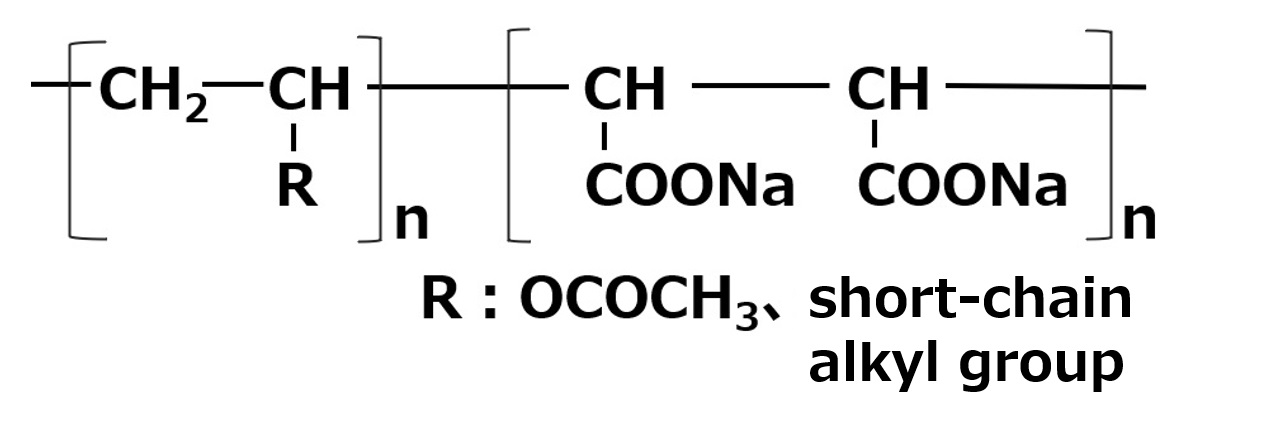 |
-Effective for dispersion of hydrophilic inorganic pigments -When R is a lipophilic group, Effective for organic pigments -Relatively high foaming |
| Aqueous dispersants | Carboxymethyl cellulose salt | 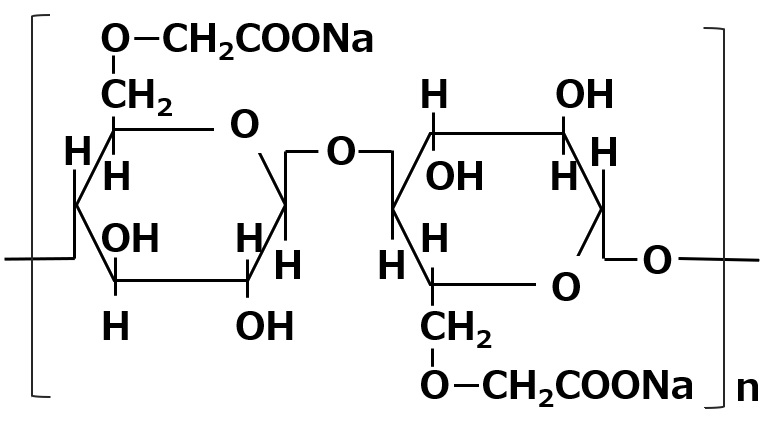 |
-Effective for dispersion of organic pigments |
| Aqueous dispersants | Polyvinyl alcohol | 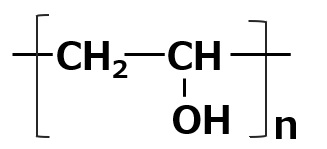 |
-Organic and inorganic pigments, etc. Effective for a wide range of pigment dispersion |
| Non-moisture dispersant | Partial alkyl esters | 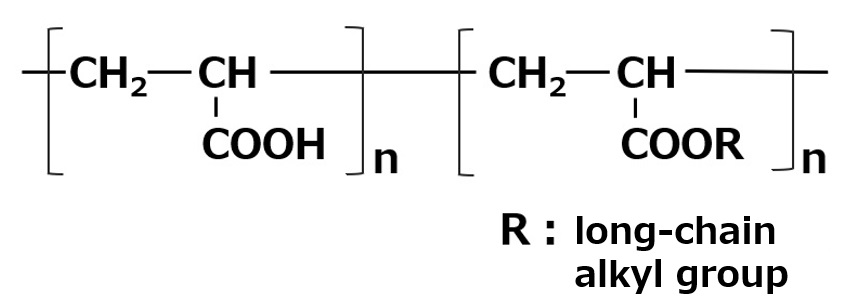 |
-Effective for dispersion of inorganic pigments |
| Non-moisture dispersant | Polyalkylene Polyamine |
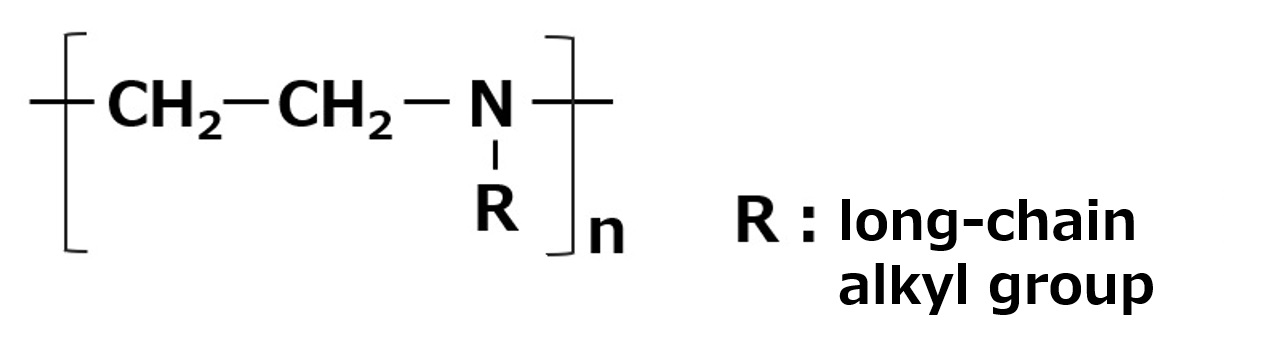 |
-Effective for dispersion of inorganic pigments |
Paints and Dispersants

For this reason, various types of pigments are used together with resins, which are the main components of coating film formation, and various dispersants are used to stably disperse these pigments.
Paint Classification
Paints can be broadly classified into aqueous paints (water-based paints) and non-aqueous paints, which can be classified into inorganic pigmented and organic pigmented types, respectively.
Non-waterborne paints are generally used for automobiles, construction, ships, machinery, woodworking, etc. Non-waterborne paints are generally used in fields where water resistance and gloss are required.
Waterborne paints are composed mainly of resin emulsions and inorganic pigments. In the dispersion process, dispersing agents, thickeners to help the dispersing agents work, anti-sagging agents, and antifoaming agents are added, and in the formulation process, additives such as antiseptic agents and antifoaming agents are added. Inorganic pigments consist of an agglomerate (secondary particles) of several to several dozen fine particles ranging from 0.01 to several microns in diameter.
In the dispersion process, the inorganic pigment agglomerates are dispersed into fine particles (primary particles), which are then mixed to produce the paint. The vivid colors of paints are due to the dispersion of pigment particles as microparticles.
What would happen if dispersants were not added in the paint manufacturing process?
First, the dispersion of pigments by the machine becomes less efficient, requiring a longer time for dispersion and placing a greater load on the machine.
Second, the color of the paint becomes dull because the dispersed particles re-agglomerate. Also, due to the precipitation of agglomerated particles, the paint can separate.
To prevent these problems, paint dispersants are used.
Inorganic pigment dispersants for waterborne paints
Inorganic pigments with high hydrophilic properties, such as calcium carbonate, titanium dioxide, and clay, are used in waterborne paints. To disperse these pigments in water, dispersants with a structure that easily blends with hydrophilic surfaces, especially polymer-type dispersants, are effective.
Typical examples of inorganic dispersants for waterborne coatings
-Sodium polyacrylate (low foaming and excellent dispersibility of many pigments)
-Copolymer of diisobutylene and maleic acid (good compatibility with resin emulsions and excellent anti-sagging property of paints)
-Condensed naphthalene sulfonic acid
These dispersants are sometimes used in combination with low-foaming nonionic surfactants that have excellent wetting properties.
For pigments with surfaces that are relatively difficult to wet, such as talc, poly(styrenesulfonic acid)-type polymers, which have both low polarity and water-soluble portions in their molecular chains, provide good dispersing effects.
Organic pigment dispersants for waterborne paints
| Pigment | Optimal HLB |
|---|---|
| Bon Red Dark | 6~8 |
| Toluisin red medium | 8~10 |
| Toluidine yellow | 9~11 |
| Phthalocyanine Green (yellowish) | 12~14 |
| Phthalocyanine Green (blue flavor) | 10~12 |
| Phthalocyanine Green (reddish) | 11~13 |
| Phthalocyanine Green (between red and green tones) |
14~16 |
| Phthalocyanine Green (between red and green tones) |
14~16 |
| Green gold | 11~13 |
| Quinacridone violet | 11~13 |
| Quinacridone red | 12~14 |
| Highly pigmented azo yellow | 13~15 |
Reference: Zenzo Seki, "Printing Guide," p10, Seibundo Shinkosha (1971)
Organic pigments and optimal HLB
In this case, dispersants with polycyclic structures such as benzene and naphthalene rings in their molecules, which have high affinity with organic pigments, are used.
For example, EOA (ethylene oxide adduct) of styrenated phenol is used. Nonionic surfactants with ester bonds are widely used to improve dispersibility by increasing the wettability of pigments.
These dispersants are selected and adjusted to achieve the optimum HLB for the organic pigment to be dispersed (left table). Anionic surfactants may also be used in combination to enhance dispersion stability through electrostatic repulsion.
In general, paint manufacturers use their own proprietary methods to combine several dispersants with different HLBs to produce coatings with improved color consistency.
Pigment dispersants for non-aqueous paints
In the case of non-aqueous paints, dispersants are less frequently used because the resin component of the paint acts as a dispersing agent, or because the pigments are modified on the surface to facilitate dispersion.
However, since paint itself is a complex system consisting of many components, alkylene polyamine surfactants and metal soaps are used as a type of dispersant to achieve other functions, such as thickening, anti-sagging, color separation, and leveling.
It is difficult to describe the relationship between the composition of dispersants and their effects in a few words, but basically, the adsorption of dispersants on the surface of pigment particles determines the performance of various paints. The table below summarizes the effects of pigment dispersants by process.
Effects of non-aqueous pigment dispersants
| Manufacturing process | Effect | Specific examples of dispersants |
|---|---|---|
| At the time of paint production | -Improvement of pigment wetting and dispersibility -Reduction of viscosity of dispersion system -Reduction of dispersion time -Vivid colors |
-Metal soap -Polyhydric alcohol fatty acid esters |
| When storing paint | -Improved pigment dispersion stability -No pigment agglomeration -No discoloration of paint |
-Metal soap -Sulfosuccinate anions |
| During the formation of the coating film | -Improvement of pigment redispersibility -Improvement of color mixing of paint film -No color lift |
-Metal soap -Fatty acid esters |
Printing Inks and Dispersants
Printed matter covers a wide range of materials, including paper, metals, textiles, ceramics, and plastics. Each of these is printed in a wide variety of ways, using printing inks and printing methods that suit the purpose and application of the printed material.
Types of printing inks
Printing methods can be broadly classified into four types according to plate type: letterpress, intaglio, planographic, and digital duplicating (see figure below). The table below shows the main types of printing methods.
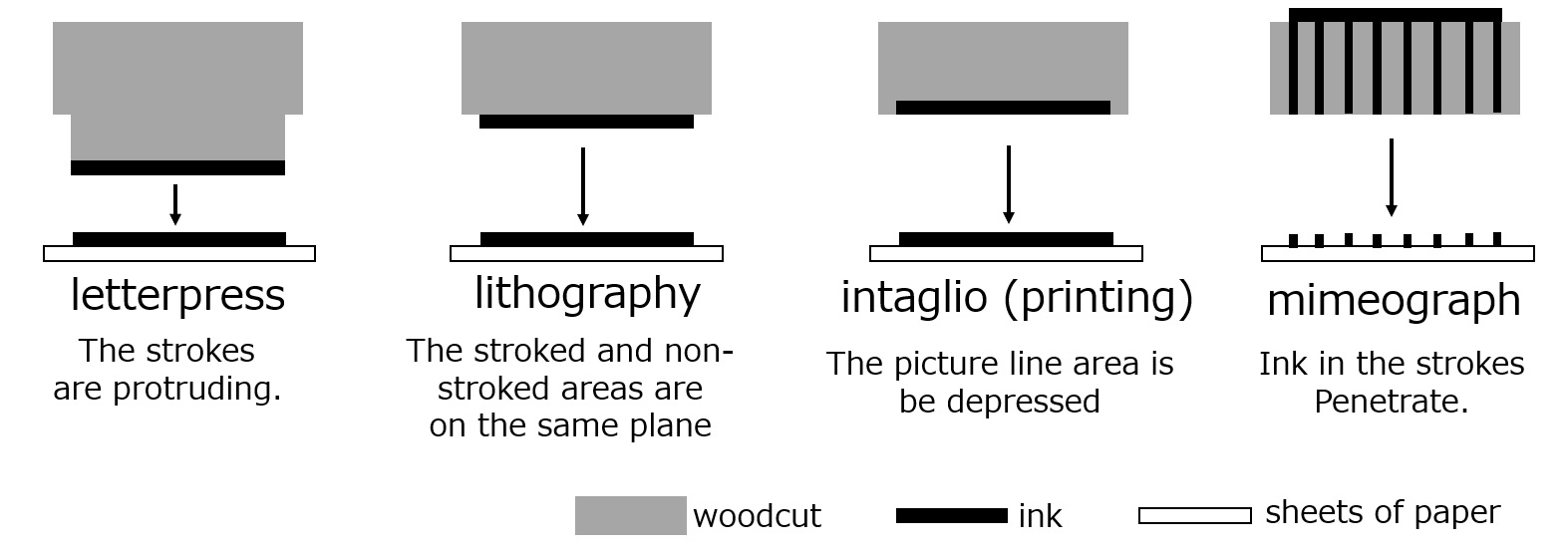
Fig. Types of illustrations (Reference: Zenzo Seki, "Printing Guide," p. 9, Seibundo Shinkosha (1971))
Printing type
| Type | Printing method | Typical printed materials |
|---|---|---|
| Letterpress | Printing | Business cards, book text |
| Stereotype | Newspaper and book text | |
| Lithography | Offset | Magazine covers, catalogs, posters, photo books, albums |
| Intaglio (printing) | Gravure | Magazine frontispieces, etc. |
| Special Rotogravure | Plastic film and metal foil packaging materials | |
| Engraving intaglio | Banknotes and securities | |
| Mimeograph | Silk screen | Posters, standing signs, pottery painting |
| Stencil | Teaching material print |
Basic composition of printing inks
The basic composition of printing inks consists of (1) pigments, (2) carriers that transfer and adhere the pigments to the substrate, and (3) auxiliaries that assist the functions of the carriers, of which dispersants are one type. The table below shows the raw materials that make up printing inks.
Raw materials that make up printing inks
| Colorants | Pigment | Inorganic pigments |
| Organic pigments | ||
| Dyes | Oil-based dyes | |
| Disperse dyes | ||
| Other dyes | ||
| Carrier | Oil | Vegetable oil (dry, semi-dry, non-dry) |
| Processing oil | ||
| Mineral oil | ||
| Binder | Natural resin | |
| Natural product derivative | ||
| Synthetic resins | ||
| Solvent | Hydrocarbon | |
| Alcohol | ||
| Glycol and its derivatives | ||
| Ester | ||
| Ketone | ||
| Other solvents | ||
| Plasticizer | ||
| Aids (additives) |
Wax | Natural wax |
| synthetic wax | ||
| Dryer | ||
| Other | (Dispersants, lubricants, cross-linking agents, gelling agents, thickening agents, anti-peeling agents, stabilizers, matting agents, antifoaming agents, color separation inhibitors, photoinitiators, antifungal agents, etc.) | |
Required viscosity of printing inks
Printing inks require pigments to be dispersed to a low viscosity to facilitate printing. The required viscosity varies depending on the printing method.
-In the case of gravure ink: A low viscosity liquid of 1 Pa・s or less is required to make it easier for the ink to penetrate into the screen cells of the plate.
-In the case of flat plate ink: A slightly higher viscosity liquid of 1 Pa・s to 100 Pa・s is required because the ink is transferred between many rollers.
In addition, printing suitability is affected by differences due to post-processing methods and printing machines, and must be optimized under these conditions. Therefore, it is necessary to select and formulate pigments, carriers, and auxiliaries to adjust and optimize their viscosity and wettability according to each printing method.
Offset ink
In recent years, offset printing has been improving image reproducibility and increasing speed, and has also come to be able to produce low-cost, high-quality printed materials in a short time with simple operations. Offset inks are printed with the thinnest film thickness of all inks, so the inks must be able to use pigments with strong coloring power in high concentration.
Principle of Offset Printing
Offset printing uses a flat printing plate with no irregularities and is composed of a hydrophilic drawing area and a hydrophilic non-printing area.
First, a fountain solution is supplied to the plate surface, covering the hydrophilic non-graphic areas with a film of fountain solution. Next, oil-based ink is supplied.
At this time, the ink is repelled by the fountain solution film on the non-graphic area, and ink does not adhere to the non-graphic area, but only to the lipophilic area, thus reproducing the image
Offset Ink Requirements
In offset printing, optimization of ink and fountain solution is an important point to ensure that the thickness of the fountain solution is appropriate, that there is no adhesion of the fountain solution to the ink surface, and that the ink does not leach into the fountain solution.
In offset inks, dispersants are rarely used. In many cases, resin components (binders) are used as dispersants or pigments are surface-treated to improve dispersion. In offset printing, pigment dispersion is achieved by wetting the pigment with the appropriate polarity of the resin, blending it in large quantities, and stabilizing the dispersion through the steric hindrance of polymers.
Main resins used for binders
-Modified alkyd resin (high polarity)
-Rosin-modified phenolic resin (excellent drying and emulsification properties)
Gravure ink
Gravure printing is one type of intaglio printing, used for publications such as magazines and posters, food packaging, and construction materials such as wallpaper and decorative laminates, and produces crisp colors and heavy printed materials. Since low-boiling-point solvents are used in the ink, it is characterized by its quick-drying properties, and is used not only for paper, but also in a wide range of fields, including plastics, metals, and composite sheets.
Principles of Gravure Printing
(1) A plate cylinder made by burning, corroding, or engraving a photograph or other image on a copper-plated cylinder is rotated while immersed in an ink pan.
(2) When the ink is pumped out and scraped off with a doctor blade, ink remains in the indentations of the strokes.
(3)This ink is transferred to the substrate with the help of the pressure cylinder to reproduce the image.
Gravure Ink Requirements
In gravure printing, ink is not transferred to the substrate by printing pressure alone, as in offset printing, but is strongly absorbed by the capillary action between the plate cylinder and the substrate. Therefore, the degree of adhesion to the plate cylinder and the smoothness and flexibility of the substrate are key points for ink transfer.
In this case, surfactants are often used as auxiliaries to improve pigment dispersion. In other words, surfactants adsorb onto the pigment surface and lower the interfacial energy, making the pigment surface easier to wet with organic solvents. In particular, since many organic pigments are not wettable, it is better to use a surfactant rich in wettability in combination with a surfactant to obtain good dispersion.
Surfactants suitable for gravure inks
-Fatty acid esters of polyhydric alcohols
-Polyoxyethylene-polyoxypropylene block polymers, etc.
Paper and Dispersants
Paper is used for a wide variety of purposes, including newsprint, tissue paper, and copy paper, making it an indispensable part of daily life. This section focuses on coated paper, which is particularly familiar with dispersion.
Coated paper
When paper is viewed under a microscope, the fibers overlap to form an uneven surface. Coated paper, also called coated paper, is made by coating the surface of paper with paint. The surface is covered with the coating to give it a beautiful surface. Coated paper is used for color printed flyers, posters, book covers, and photo magazines.
Coated paper has developed rapidly in recent years along with the development of printing technology, and in order to obtain a more beautiful printed product, a paper with a smoother surface and better ink transferability is being sought.
Coated papers are classified according to the amount of coating applied to the paper as shown in the table below.
Classification of coated paper
| Type | Coating amount | Use |
|---|---|---|
| coated paper | One side 20g/m2 | Fine art photography books, etc. |
| coated paper | One side 10g/m2 | Posters, calendars, etc. |
| medium quality coated paper | One side 20g/m2 | Newspaper flyers, photo weeklies, etc. |
| Lightweight coated paper | One side 5g/m2 | Newspaper flyers, photo weeklies, etc. |
| micro-coated paper | Double-sided 5g/m2 | Newspaper flyers, photo weeklies, etc. |
Coating solution
Coated paper used as printing paper is made by applying a pigment dispersion called a coating color to the surface of the paper, and the pigment dispersion can be considered a kind of paint. Clay and calcium carbonate are mainly used as pigments, and titanium dioxide and satin white are also used.
Coating solution manufacturing procedure
(1) Add a predetermined amount of pigment to water in which an appropriate amount of dispersant has been dissolved in advance, while stirring with a dispersing machine.
(2) Add a water-soluble binder such as starch and synthetic latex such as SB latex while continuing stirring.
(3) Add a waterproofing agent, lubricant, antifoaming agent, preservative, etc.
The resulting coating solution is filtered and sent to the coating station, where it is applied to the paper, the surface is smoothed, dried, and finished with a surface finishing machine called a super calender.
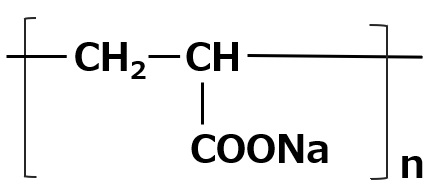
Dispersants for coating liquids
As dispersing agents, natural polymers such as casein and gum arabic, and complex phosphates such as sodium hexametaphosphate have been used in the past, although this is influenced by the type of pigment. Currently, polycarboxylic acid polymers (e.g., polyacrylates) are most commonly used for their dispersing effect.
These polymers are adaptable to various dispersant needs by changing the average molecular weight, type of neutralizing salt, and copolymerization ratio with other monomers. A recent trend is the shift to high-speed coating from the viewpoint of productivity improvement. In order to further increase the concentration of coating solution, there is a growing demand for dispersants that can reduce viscosity, and development of such dispersants is ongoing.
In many cases, pigments used in coating solution are fed into a pigment slurry with a dispersing agent in advance. In this case, as in the case of coating liquids, there is a growing demand for dispersants that can further increase the concentration of pigment slurry and reduce its viscosity in order to enable high speed coating.
Cosmetics and Dispersants
Types and composition of cosmetics
The types of makeup cosmetics and their raw materials are shown in the table below.
First, the key to manufacturing cosmetics is to disperse these pigments finely into primary particles and uniformly disperse them in a dispersing medium. If agglomeration occurs, colors may become dull or uneven. Next, stability over time is important. Poor stability may cause color separation, pigment sedimentation, or gelation. For this reason, the following points should be considered
Dispersion considerations for cosmetics manufacturing
(1) Before or during the formulation of cosmetics, reduce the particle size of pigments and achieve a sharp particle size distribution.
(2)Select a dispersant that is close to the specific gravity of the pigment. (At this time, you can also add polymers or swollen viscosity substances to increase the viscosity of the dispersant.)
(3) Use ionic surfactants to create an adsorption layer on the pigment surface to increase the surface potential and repulsion force.
(4) Use a surfactant that improves the wettability of pigments with dispersants in combination with an ionic surfactant.
(5) Adsorb polymers such as cellulose derivatives onto the pigment to create a protective colloidal layer.
Ingredients used for foundation and foundation powder

| Type | Raw materials | |
|---|---|---|
| Base | Pigment | |
| Fine powder | Metallic soap, body pigment | Organic and inorganic pigments |
| Solid | Fats, oils, waxes, fatty acid esters, hydrocarbons, surfactants, Metallic soaps, polymer compounds, body pigments |
pigments, Pearl pigments |
| Emulsification type | Fats, oils, waxes, fatty acids, higher alcohols, fatty acid esters, hydrocarbons, surfactants, metallic soaps, polymer compounds, inorganic thickeners, polyhydric alcohols, body pigments, purified water |
Organic and inorganic pigments |
| Oil-based | Fats, oils, waxes, fatty acid esters, hydrocarbons, surfactants, Polymer compounds, body pigments | Organic and inorganic pigments |
Ingredients used in lipstick
| Raw materials | ||
|---|---|---|
| Type | Base | Pigment |
| Stick | Fats, oils, waxes, higher alcohols, fatty acid esters, hydrocarbons, surfactants, body pigments | Organic pigments, inorganic pigments, pearl pigments |
| Gloss | Fats, oils, waxes, hydrocarbons, surfactants, body pigments | |
Ingredients used for eyeliner
| raw materials | ||
|---|---|---|
| Type | base | pigment |
| Oil-based | Fats, oils, waxes, fatty acid esters, hydrocarbons, surfactants, body pigments | Inorganic pigments |
| Volatile oil type | Fats, oils, waxes, fatty acid esters, hydrocarbons, surfactants, polymer compounds, inorganic thickeners, Volatile oils (solvents), body pigments |
|
| Emulsification type | Fats, oils, waxes, fatty acids, higher alcohols, fatty acid esters, hydrocarbons, surfactants, metallic soaps, Polymer compounds, inorganic thickeners, polyhydric alcohols, body pigments, purified water |
|
| Emulsion polymer type | Surfactants, metallic soaps, polymer compounds, inorganic thickeners, polyhydric alcohols, body pigments, purified water | |
| pencil shape | Fats, oils, waxes, fatty acids, higher alcohols, fatty acid esters, hydrocarbons, body pigments | |
Water-based cosmetics
Typical cosmetics in which pigments are dispersed in aqueous systems include water oshiroi and eyeliner. Nonionic surfactants, especially ester surfactants, are effective dispersants.
| Ingredients | Amount (by mass) |
|---|---|
| pigment | 15 |
| deionized water | 129 |
| glycerin | 5 |
| thickener | 1 |
| total amount | 150 |
Example of eyeliner formulation
Low-viscosity pigment dispersion systems such as eyeliner require the use of water-soluble polymers or swollen viscosity substances in combination for thickening.
Excellent water-soluble polymers include cellulose derivatives such as carboxymethyl cellulose, synthetic polymers such as polyvinyl alcohol and polyvinyl acetate, and inorganic thickening agents such as Mg-Al silicate.
Non-aqueous cosmetics
Lipstick, oil-based foundations, eyebrow pencils, and nail enamel are examples of cosmetics in which pigments are dispersed in a non-aqueous system. Lipsticks, for example, are often dispersed in a molten state by applying heat, placed in a mold, and allowed to cool and harden. The key is to make the pigment surface hydrophobic and close to the polarity of the solvent. By using a surfactant of the opposite ion to the colloidal particles of the pigment as a dispersing agent, a monolayer is formed on the pigment surface, making the pigment lipophilic (hydrophobic).
Anionic surfactants and sulfosuccinate types are good dispersants. Other methods to stabilize dispersion include the use of natural waxes and surface treatment of pigments to change their hydrophilicity from hydrophilic to hydrophobic.
| Ingredients | Amount (by mass) |
|---|---|
| polyethylene wax | 8 |
| cecylene wax | 5 |
| candelilla wax | 2 |
| liquid paraffin | 40 |
| glycerin triisostearate | 40 |
| Red No. 202 | 4 |
| iron oxide black | 0.5 |
| titanium dioxide | 0.5 |
| total amount | 100.0 |
Example of lipstick formulation
An example of a lipstick formulation is shown on the left.
Cement and dispersants
Concrete is a material consisting of fillers such as sand and gravel bound together by a type of inorganic adhesive called cement.
Cement, which plays an important role in the composition of concrete, is made by firing limestone, clay, or gypsum at approximately 1,500°C. The resulting cement reacts with water and hardens. The resulting cement reacts with water and hardens, acting as an adhesive to hold together the sand and gravel that are mixed in at the same time to form concrete. The composition of a typical Portland cement and its reaction products with water are shown in the table below.
Composition of Portland cement and reactants with water
| Composition of Portland cement | Reaction products with water | |
|---|---|---|
| 1) CaSO4・2H2O (gypsum) 2) 3CaO・Al2O3 (Calcium aluminate trioxide) |
H2O → |
3CaO・Al2O3・3CaSO4・32H2O 3CaO・Al2O3・6H2O |
| 3) 3CaO・SiO3 (Calcium trioxide silicate) 4) 2CaO・SiO2 (Calcium dioxide silicate) |
H2O → |
3CaO・SiO2・3H2O Ca(OH)2 |
| 5) CaO・Al2O3・Fe2O3 (Calcium aluminoferrate trioxide) | H2O → |
3CaO・Al2O3・6H2O 3CaO・Fe2O3・6H2O |
Role of cement dispersant (water reducer)
The strength of concrete tends to decrease as the amount of water is increased. However, as the amount of water is reduced, the fluidity of the concrete deteriorates and workability worsens. The theoretical amount of water required for cement to hydrate and solidify is usually 20-25% of the cement mass. On the other hand, to mix concrete to a workable level, 40-60% of water is required relative to the mass of the cement.
This is where dispersants (water-reducing agents) come in: they are added to cemento water-mixture systems to improve the dispersion of cemento particles in water, give fluidity even with small amounts of water, and contribute to improved workability and concrete strength.
In some cases, it takes several hours or more after the concrete mixture is mixed at the plant before it is used at the construction site. In such cases, the viscosity of the concrete slurry increases over time, making it difficult to handle and, in extreme cases, impossible to work with. This can be a major problem especially in cities with poor road conditions and heavy traffic congestion.
Again, a dispersing agent (fluidizing agent) that can maintain the initial fluidity is needed. The role of cement dispersants in the manufacture and use of ready-mixed concrete is shown in the table below.
Role of cement dispersants in the manufacture and use of ready-mixed concrete
| Kneading process | Filling and transportation process | construction |
|---|---|---|
| Materials: cement, aggregate, water Additive: Water reducer |
Filling concrete mixer trucks and transporting to construction sites | Constructed at construction site Additive: Fluidizing agent |
Cement dispersant type
Dispersing agents for cement can be classified into two categories according to their intended use: water reducers and fluidizing agents. In order to create strong concrete structures, the amount of water used for mixing must be reduced as much as possible. Therefore, water reducers, which are dispersants for cement, are used in ready-mixed concrete production plants and concrete product manufacturing plants.
Superplasticizer
Concrete products (poles, piles, hume pipes), concrete blocks for seawalls, and sleepers require even higher concrete strength than general-purpose concrete construction. When used in these applications, air bubbles must be removed to further increase the density or further reduce the amount of water. In this case, a dispersant that is more effective in reducing water and removing air bubbles must be used. This water reducer is specifically called a high-performance water reducer.
AE water reducer
AE (Air Entraining), also known as air entraining, is a property of ready-mixed concrete that allows air bubbles to be trapped within the concrete. When water left inside a concrete structure freezes and thaws during the winter and this process is repeated, the concrete cracks and its strength is significantly reduced. However, adding air bubbles to ready-mixed concrete has the effect of preventing this from happening.
Typical cement dispersants
Lignin sulfonic acid type
The oldest dispersant in use, it is made by denaturing lignin sulfonic acid, which is generated in the sulfite pulp manufacturing process. Although it is not very effective in reducing the amount of water added, it is often used as an AE reducer in the construction of general concrete structures because it is inexpensive and has AE properties as well.
Naphthalene sulfonate, melamine sulfonate
These water reducers are used as high performance water reducers because they have a greater water reduction effect than lignin sulfonate reducers. They are also used as fluidizing agents because they improve the fluidity of concrete when added in small quantities. Melamine sulfonate is used especially for structures where aesthetics are important, as it gives the finished product a beautiful surface.
Polycarboxylic acid type
Compared to the above water reducers, the effect of water reduction is not so great, but it is characterized by its ability to control the increase in concrete paste viscosity for a relatively long period of time. Therefore, it is best suited as a fluidizing agent, and is often used in combination with other water reducers or high-performance water reducers.
Molecular weight of dispersants for cement
In general, polymers are more advantageous as dispersants than low-molecular surfactants, but there is an optimum molecular weight; for example, naphthalenesulfonic acids have a molecular weight of 2,000 to 3,000, and polycarboxylic acids have a molecular weight of 5,000 to 10,000.
If the molecular weight becomes too large, the molecules adsorbed on the cement surface form long dangling chains, which entangle with each other, increasing the dispersion viscosity and deteriorating flowability. Also, when the molecular weight becomes extremely large, the agglomeration effect takes precedence over dispersion.
How Cement Dispersants Work
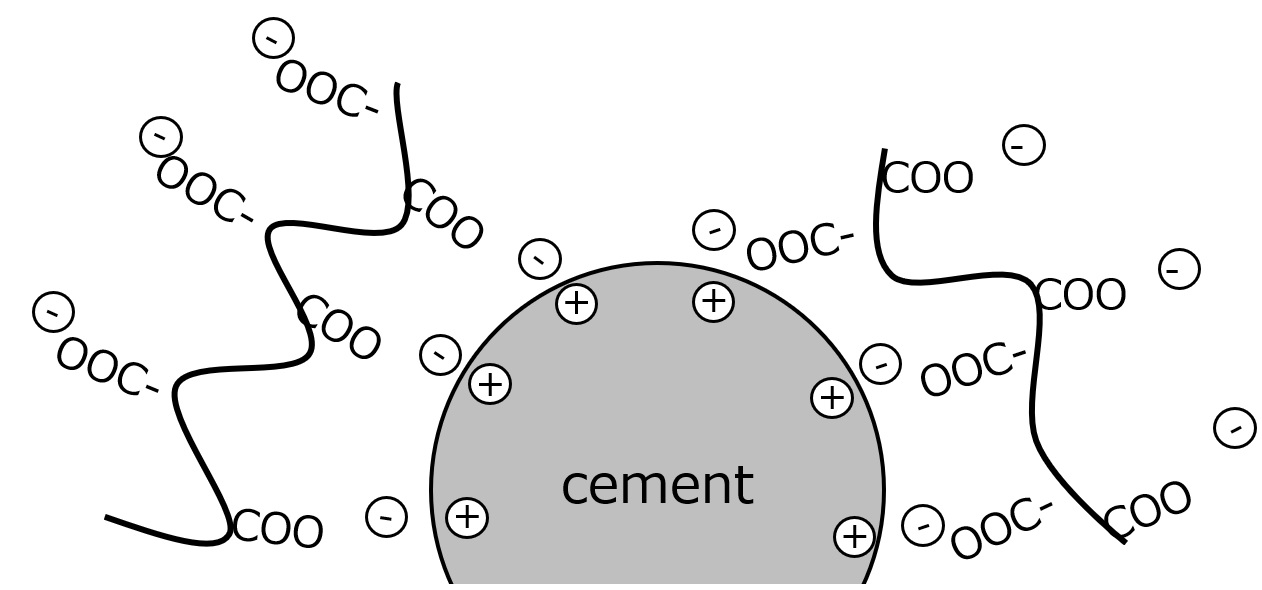
Fig. Adsorption of dispersant on cement surface
Pesticides and dispersants
Pesticides are used for fungicidal, insecticidal, and herbicidal purposes. Most bulk agrochemicals are either insoluble or insoluble in water, and are available in solid and liquid forms.
In general, pesticides are used in the form of an active ingredient plus an auxiliary agent in order to achieve uniform application of a relatively small amount of the active ingredient over a large area. This API plus an auxiliary agent is called a formulation. Formulations are classified according to the form used, as shown in the table on the right.
Among these, those that are dispersant-related include hydration agents, flowables, and granules.
Hydrating agent
Hydrates are used as insecticides for paddy rice, vegetables, and fruit trees. They are formulated by adding talc, kaolin, and other ingredients to an agrochemical base of several micrometers in size, and then adding surfactants and water-soluble polymers.
The function of the dispersant is to make the product wettable in water, to disperse it stably, and to easily re-disperse by agitation any product that separates or settles after being left to stand.
Flowables
Used in insecticides for paddy rice, vegetables, and fruit trees, this formulation is made by microparticulating the agrochemical agent and dispersing it in a dispersant such as water using a surfactant. In order to maintain the dispersion for as long as possible, water-soluble high molecular weight or inorganic thickening agents are often added.
Advantages of flowables
The advantages are that it can be made into a liquid formulation of the original substance, which cannot be emulsified due to lack of suitable organic solvents, and because it does not use organic solvents, there is no risk of chemical damage or flammability caused by organic solvents.
Disadvantages of flowables
They are usually only able to produce low-concentration dispersion systems of 50% or less, which leads to low productivity and high transportation costs. The wet milling method of production also leads to higher costs.
granule
Agrochemical granules are made by mixing an agrochemical API with an inorganic carrier such as bentonite, a binder, and a dispersant such as a water-soluble polymer, and then granulating them (0.5 to 1.5 mm in diameter and 3 to 10 mm in length) in a granulator. An example of agrochemical granule production is shown in the figure below.
Features of Granule Formulations
Unlike powders and emulsions, granules do not form fine powder or droplets when sprayed, making them highly safe. The spraying method is also easy.
Types of agricultural formulations
| Formulation properties | Formulation name | Usage | |
|---|---|---|---|
| Solid-state | Powder | Regular powder | Spray as is |
| DL Powder | |||
| Flow dust | |||
| Granule | 1 kg granule | ||
| Powdered granule | Fine granule | ||
| Fine granule F | |||
| Micro granule F | |||
| Hydrating agent | Dilute with water Spray |
||
| Granule hydrating agent (WDG, WG,dry flowables) | |||
| Aqueous solution | |||
| Other | Tablets and powders | ||
| Liquid | Emulsion | Spray as is Diluted with water Spray |
|
| Liquid | |||
| Oil-based agent | Surfing agent | Spray as is | |
| Flowable (SC, FL) | Spray as is | ||
| Emulsion (EW) | Diluted with water Spray |
||
| Microemulsion (ME) | |||
| Saspo emulsion (SE) | |||
| Microcapsules (MC, CS) | |||
| Other | Aerosol, paste agent, Smoking agent, WSBs (throw-in agents), Fumigant, Coating agent |
ー | |
Granule requirements
When granules are applied to a paddy field, for example, they must be able to disintegrate quickly and spread quickly in the water (disintegrative spreadability).
Pesticides suitable for granular formulations must have properties that allow the active ingredients to dissolve in water to some extent and to penetrate into the plant body. From this point of view, insecticides and herbicides are often used in granular formulations, and granular herbicides account for 80% of all herbicides.
In recent years, the development and formulation of systemic fungicides has also progressed. Here we focus on granular formulations, which have been steadily increasing in production in recent years and are also produced in large quantities.
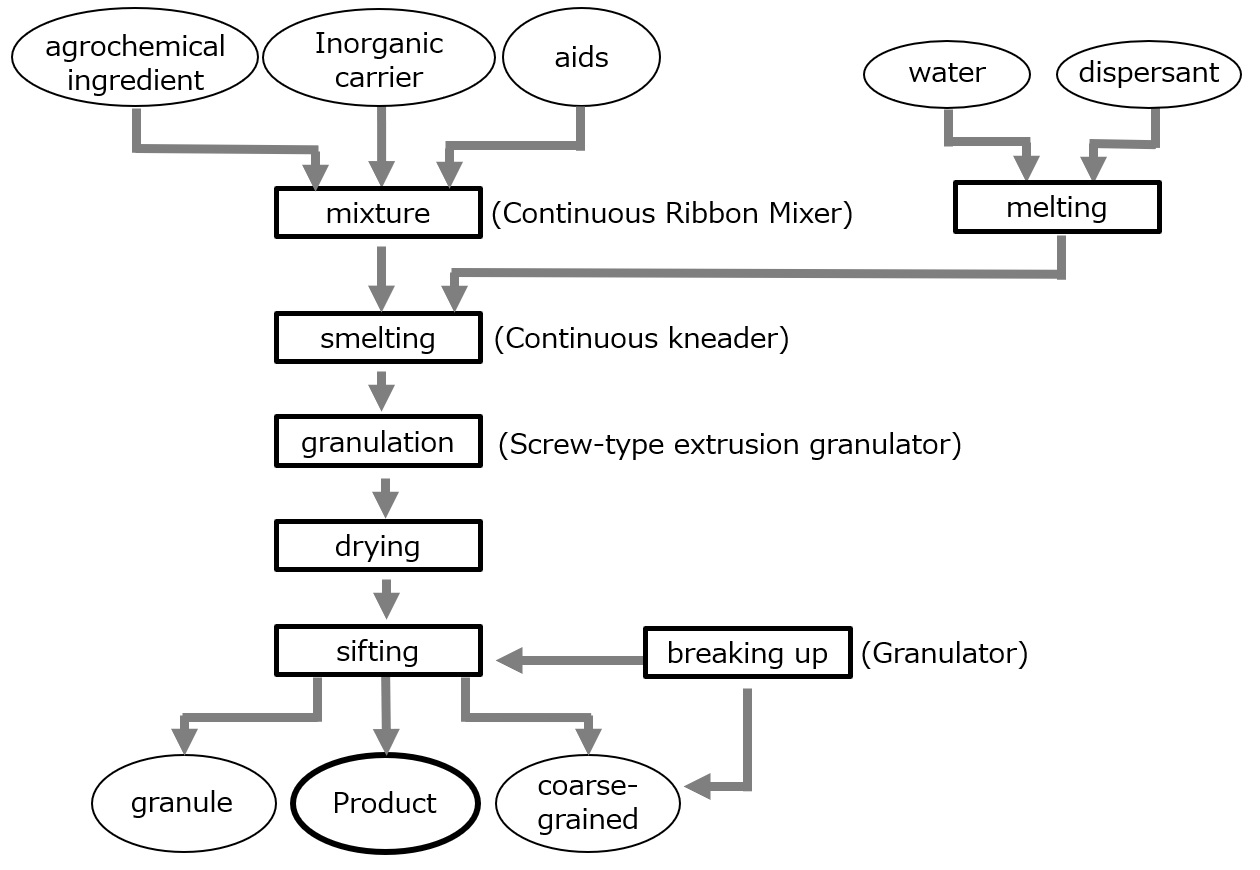
Fig. Production flow of agrochemical granulation by extrusion granulation method
Granule type
Example of standard formulation of agrochemical granules
Examples of standard formulations of agrochemical granules are shown in the table to the right.
Inorganic carriers
Bentonite, talc, clay, kaolin, calcium carbonate, etc.
Binders
Polyvinyl alcohol, sodium carboxymethyl cellulose, gelatin, etc.
Dispersants
The following polymers are generally used.
-Sodium polyacrylate
-Sodium lignin sulfonate
-Formalin condensate of sodium naphthalene sulfonate
| Ingredients | Amount (%) active ingredient |
|---|---|
| prime field | 3~10 |
| Inorganic carrier | 95.5~85 |
| binder | 0.5~2 |
| dispersant | 1~3 |
Dispersant for agrochemical granules
Although the amount of dispersant used is as small as 1-3%, it plays an important role in the disintegration and spreading of the granules.
-Polyacrylic acid type polymers such as sodium polyacrylate have the best dispersing performance among them.
-Sodium lignin sulfonate is inexpensive and is often used when dispersibility is not so important.
-Formalin condensates of sodium naphthalenesulfonate have the advantage of not being affected by the hardness or pH of the water.
Granular agents manufactured in this way are generally applied at a rate of several kilograms per 1,000 meters2 of paddy field. Recently, 1 kg granules, which contain about three times as much active ingredient as the 3 kg granules and have a slightly larger particle diameter, are becoming the mainstream.
Example of Herbicide Butachlor Granule Formulation
Examples of Butachlor granule formulations used as herbicides are shown in the table to the right.
Granule production methods include extrusion granulation, adsorption, and spraying, all of which are designed to disintegrate in water.
| Ingredients | Amount (%) active ingredient |
|---|---|
| Butachlor (herbicide active ingredient) | 2.5 |
| Bentonite (inorganic carrier) | 30.0 |
| Clay or talc (inorganic carrier) | 66.0 |
| Polyvinyl alcohol (binder) | 0.5 |
| Sodium polyacrylate (dispersant) | 1.0 |
| total amount | 100.0 |
Function of dispersants for agrochemical granules
The role of dispersants for agrochemical granules is mainly to disperse the inorganic carrier with the agrochemical API adsorbed in water. In the past, low molecular weight anionic surfactants such as sodium alkylbenzenesulfonate were used as dispersants, but in recent years, water-soluble polymers such as sodium polyacrylate have become the mainstream. The reason for this is said to be that they bind strongly to the carrier and form a stable protective colloid, thereby improving dispersibility.
The figure below shows the dispersion of granules made with water-soluble polymers in water.
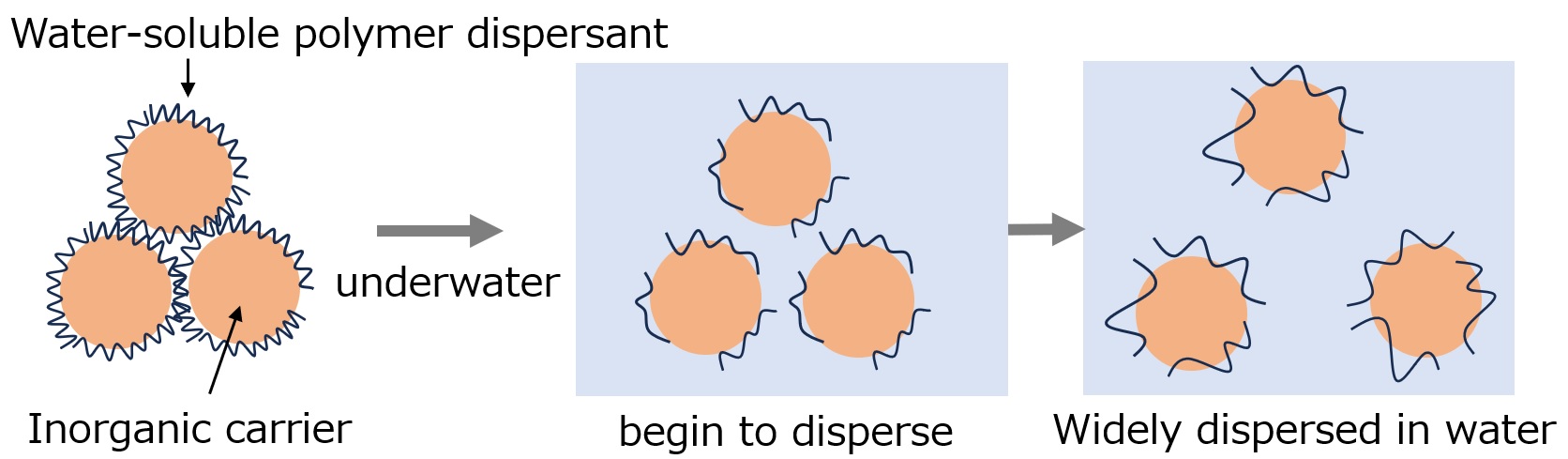
Fig. State of pesticide granules in water
Dyeing and Dispersion
The difference between dyeing with pigments and dyeing with dyes is that pigments are adhered to the fiber surface with binders in the form of primary or secondary particles to produce color, whereas dyes diffuse at the molecular level into the amorphous part of the high molecular chain that constitutes the fiber and react with or fix to the fiber to produce color.
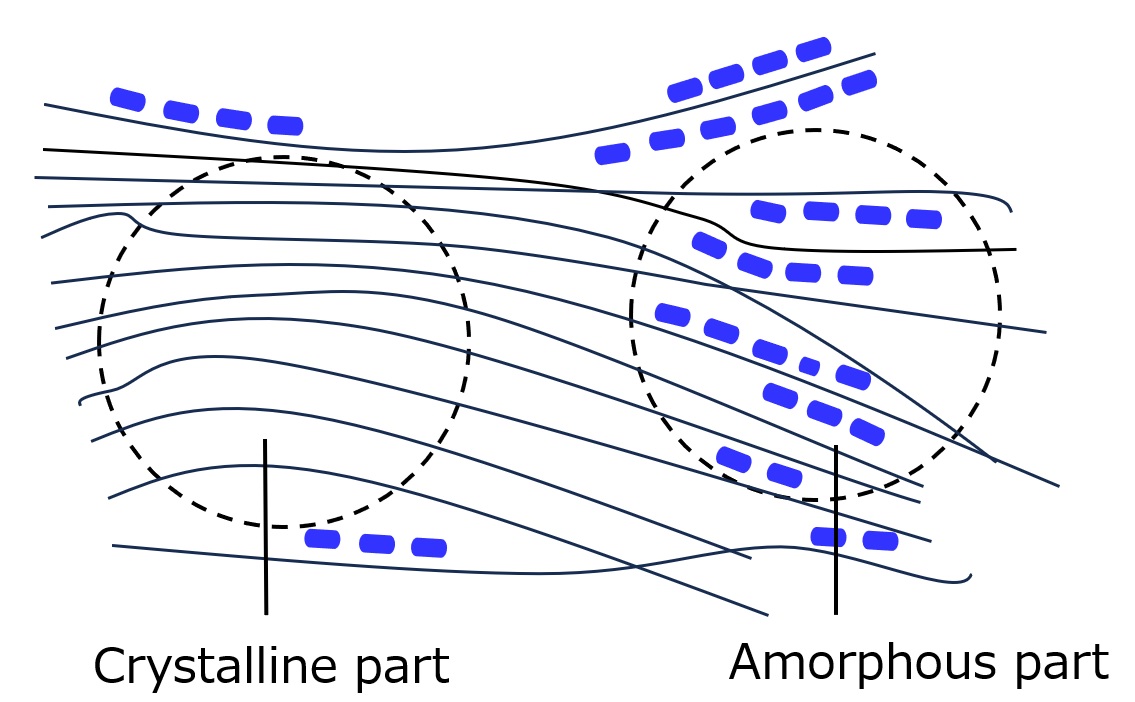
Fig. Schematic diagram of dye deposition
The left figure shows how dyes are dyed. The mechanism of coloration is the same for water-insoluble disperse dyes.
General dyes are water-soluble, so there is no dispersion problem; however, for water-insoluble disperse dyes, uniform dispersion of the dye in water is indispensable for beautiful dyeing.
This section describes disperse dyes.
Need for dispersant for disperse dye
Fibers composed of cellulose such as cotton and rayon have hydroxyl groups, while nylon and wool have amino groups and other functional groups that are hydrophilic and easy to react with, and can be dyed with water-soluble dyes that react with these functional groups. However, for fibers that are strongly lipophilic (hydrophobic) and do not have functional groups, such as polyester fibers, dyeing is performed using disperse dyes that are insoluble in water. Therefore, a dispersing agent is required to disperse the dye uniformly in water.
Commercially available disperse dyes contain about half of ionic dispersants in addition to dyes. When polyester fibers are dyed by the dip-dyeing method (a method of dyeing a single color with a water-dispersion system of dyes), a disperse dye dispersion solution is often circulated. At this time, dye particles must be stably dispersed in a temperature range from room temperature to 120~130°C. If the dispersion stability is poor, uneven dyeing or dull colors may occur, and therefore, the choice of dispersing agent is important.
On the combination of dispersants
In general, there are few dispersants that exhibit good dispersibility from room temperature to the high temperature (120-130°C) range, and they are often used in combination.
For example, formalin condensate of sodium naphthalenesulfonate (NSF) shows excellent dispersibility near room temperature, but tends to decrease dispersibility at temperatures above 50°C. NSF has no functional group in its molecule that interacts with dyes and simply provides dispersibility by physical adsorption. and dispersibility decreases above 50°C. On the other hand, metacresol sulfone has a tendency to decrease dispersibility above 50°C.
On the other hand, CSF, a formalin condensed product of sodium metacresol sulfonate, has a phenolic hydroxyl group and thus adsorbs functional groups such as -NH2 (amino group), >C=O (ketone), and -COOH (carboxyl group) on the dye surface through ionic interaction and shows excellent dispersibility at high temperature. Therefore, the combined system of NSF and CSF provides stable dispersibility from low to high temperatures.
However, dispersion of dyes is often insufficient with these dispersants alone, and surfactants are often used in combination.
-Function of non-ionic surfactant: Eliminates unevenness of dyeing color and makes dyeing uniform.
-Function of anionic surfactant: To improve dye dispersion
Dispersing and equalizing agent for polyester dyeing
In practice, dispersion equalizing agents for polyester dyeing, which are a well-balanced combination of these various surfactants, are generally used. In recent years, liquid flow dyeing machines, in which dyeing solution is sprayed from a jet nozzle and moves through the dyed cloth in contact with the cloth, have become widely used for dyeing polyester fibers. In addition to improving the dispersibility of disperse dye, foaming is often a problem, requiring a dispersing agent with low foaming characteristics.
Dyeing method for polyester
There are three industrial dyeing methods for polyester fibers:
(1) high-temperature, high-pressure dyeing, (2) carrier dyeing, and (3) thermosol dyeing.
In all cases, as shown in the figure below, polyester is dyed by loosening the molecular chains of the polyester (making the crystal structure loose).
(1) High-temperature, high-pressure staining method
This method is based on the fact that molecular motion becomes more active as temperature is raised. The dyeing temperature is set to 120-130°C to loosen the crystalline structure of the fiber and increase the gap between the fibers, allowing dye molecules to enter the fiber.
(2) Carrier staining method
This method uses a chemical called "carrier" to expand the gaps in the amorphous region of the fiber, even at dyeing temperatures of 100°C, to make it easier for the dye to penetrate.
(3)Thermosol staining method
After being padded with a dye dispersion mixed with a small amount of glue, the dye is dried and placed evenly on the surface of the fabric, which is then heated to 180 to 200°C for 30 to 60 seconds to instantaneously sublimate the dye and feed it into the fabric.
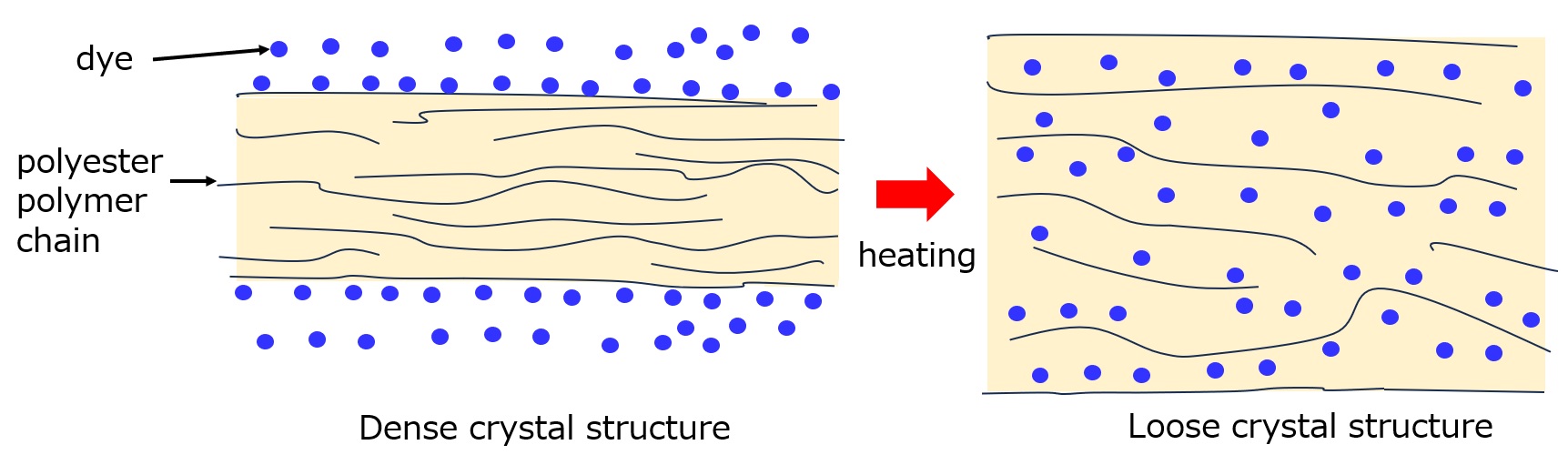
Fig. Dyeing model for polyester fiber
Function of dispersants for dispersed dyes
The dyeing process of disperse dyes is shown in the figure below. When a disperse dye is combined with a surfactant, the state of the disperse dye in aqueous solution changes as shown in the figure below.
When the temperature is raised in this state, the slightly dissolved dye enters the amorphous region of the polyester fiber, the gap of which is widened by the water, and the dyebath is devoid of the dissolved dye. At this stage, there is only a trace of color on the fiber.
Next, the dye equivalent to the solubility in the bath solution (about 5 to 10 mg per 1 L) dissolves out of the dispersed dye area as shown in the figure below. The dissolved dye then re-enters the amorphous part and is dyed. This process is repeated, and dyeing of the disperse dye proceeds.
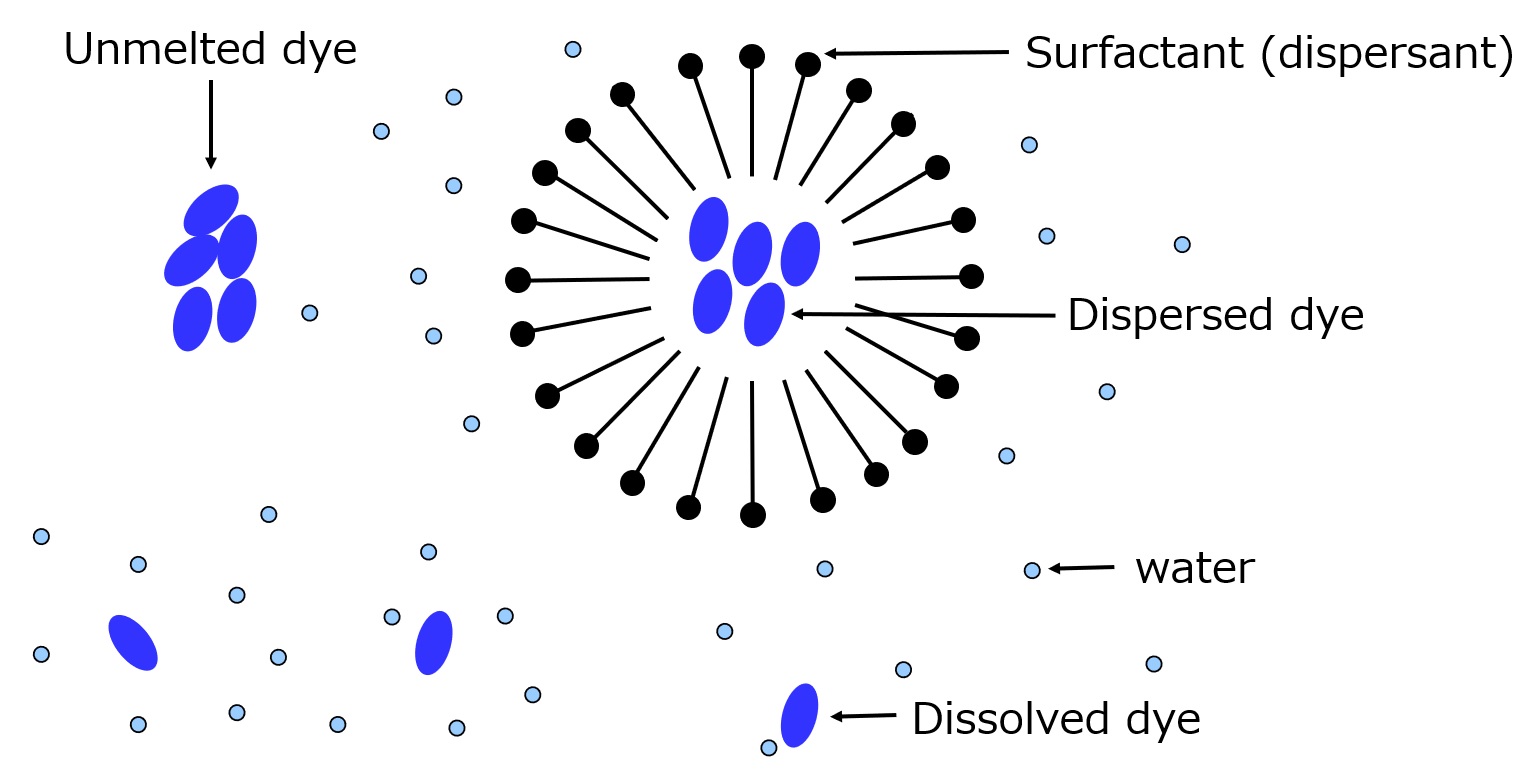
Fig. Schematic diagram with dispersed dye
Plastic coloring and dispersion
Internal coloring: Dye or pigment is kneaded into the plastic at the raw material or molding stage to achieve uniform coloring all the way to the inside.
Surface coloring: Coloring the surface by painting, printing, etc.
In general, the term "plastic coloring" often refers to internal coloring.
Plastic coloring aids and coloring methods
Pigments do not melt when heated, nor do they dissolve in common organic solvents. In addition, they are often incompatible with the properties and composition of plastics because they are very different. Therefore, it is difficult to mix pigments into plastics without re-agglomerating them as they are. Dispersants called plastic coloring aids solve this problem.
The following methods are available for coloring plastics
Dry-color process
This method adds pigments to resins as a fine powder whose surface is treated with plastic coloring aids such as surfactants or metallic soap to make it easily blend with the mating resin. Although it is used for most thermoplastic resins, it has disadvantages such as severe scattering contamination due to its dry powder nature and different coloring properties depending on the blending conditions with the raw resin.
Liquid color, paste color
In this method, pigments are dispersed finely and concentrated in plasticizers and solvents in advance, together with plastic coloring aids, and added to the resin as a dispersion. The coloring state is unstable, and the pigments may agglomerate or settle due to volatilization of the plasticizer or solvent after long-term storage.
Master batch
In this method, a master batch is made by using a resin to be pigmented or a resin that is compatible with the pigment and dispersing the pigment in high concentration together with plastic coloring aids, which are then added to the resin. This method has become popular in recent years and has no adverse effects on physical properties or contamination, and the pigments are well dispersed. On the other hand, it requires a resin for the dispersant that can blend well with the other resin.
A typical flow sheet using plastic colorants is shown in the figure below.
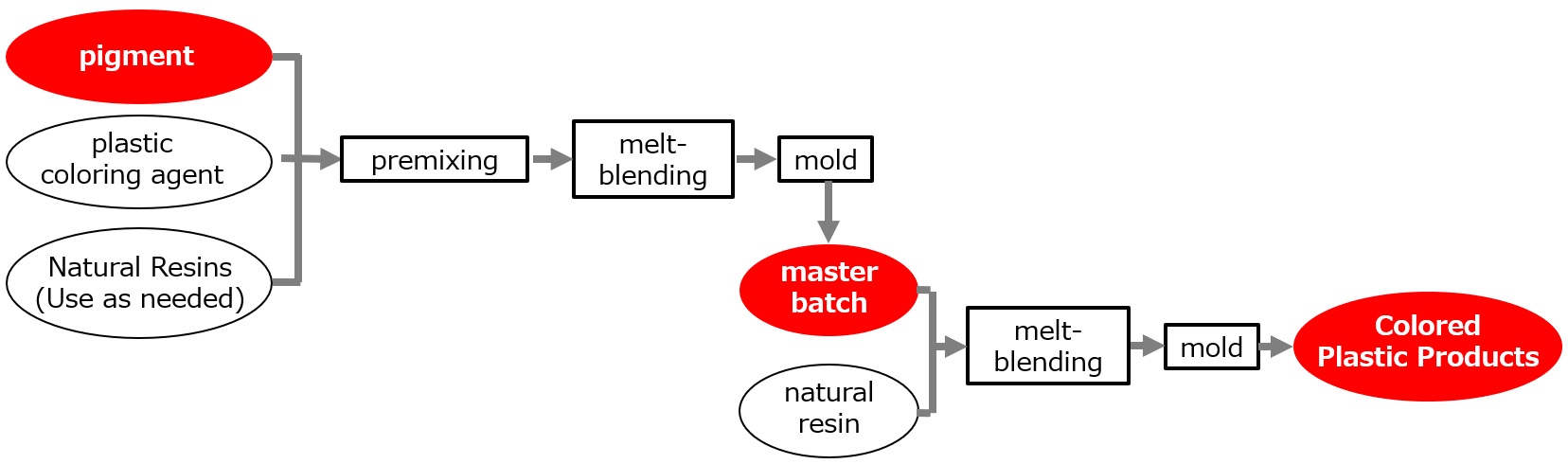
Fig. Typical production flow using plastic colorants
As mentioned above, masterbatches are colorants in which pigments are dispersed in resin at high concentration, and are available in pellet, plate, and flake forms. Pigment content is typically 30-70%.
The amount of pigment required in a final plastic product, for example polyolefin, is generally around 0.5% by mass (vs. resin). Therefore, the master batch should be mixed with 60 to 140 times as much resin. Since the same type of resin as that to be used for coloring is generally used, there are no problems in terms of physical properties, and the dispersion is excellent.
In particular, if the dilution ratio of the masterbatch to the natural resin (original resin to be colored) is too large (small amount added), uneven coloration is likely to occur.
Synthetic wax-based plastic coloring aids
For a long time, rolaffin wax, obtained by refining petroleum, has been used as a typical plastic coloring aid, but in recent years there has been a shift to synthetic wax systems, which offer superior pigment dispersibility.
-Synthetic waxes such as polyethylene wax and polypropylene wax
Used to color polyolefins such as polyethylene and polypropylene, and polyvinyl chloride
-Low molecular weight polystyrene
Used for coloring styrene-based resins such as polystyrene and ABS resins
-Modified olefin oligomer
Used to color polyethylene and polypropylene.
| Ingredients | Amount (%) |
|---|---|
| Titanium dioxide (pigment) | 40 |
| Polyethylene wax (plastic coloring agent) | 30 |
| High-density polyethylene (natural resin) | 30 |
| total amount | 100 |
Example of Master Batch Composition
The table on the left shows an example of a masterbatch composition when polyethylene wax is used as a plastic coloring aid.
How Plastic Color Auxiliaries Work
The primary particles of pigments are several microns in size, but when they are actually used, they are sometimes agglomerated to several tens of microns due to static electricity and other factors. When a master batch is made from these pigments, the pigments are again primary particles or in a state similar to this.
When this is mixed with a natural resin, the pigments that have become fine particles are transferred into the mating resin along with plastic coloring aids, resulting in a uniform dispersion. The figure below shows how a masterbatch is used to color a resin.
Plastic coloring aids have lower melt viscosity and higher affinity for pigments, which is the reason why pigments are dispersed more uniformly in plastic coloring aids than in natural resins.
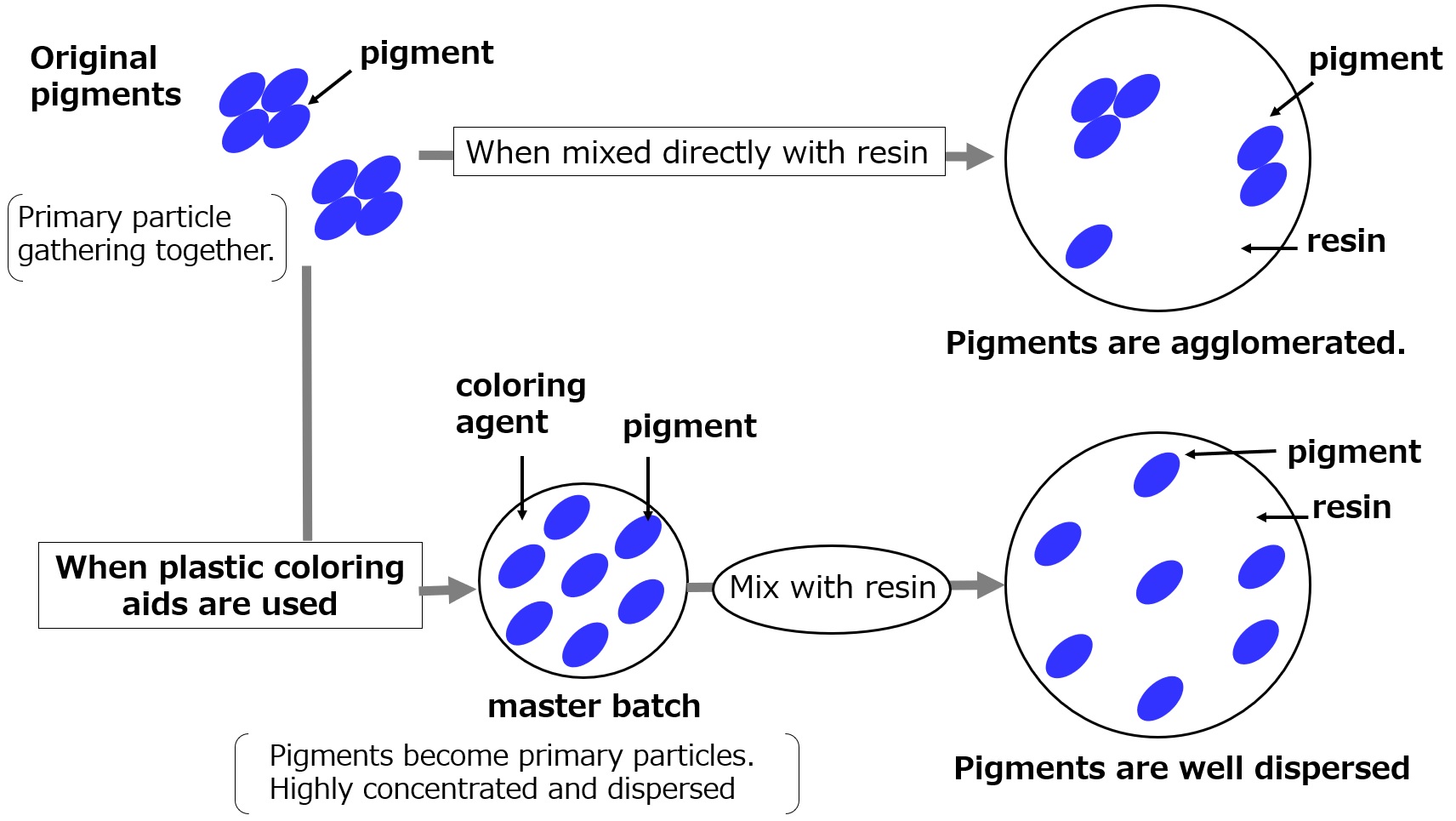
Fig. Coloring of resins using masterbatches with plastic coloring aids
Related Information
Related products




Topics
| Links to Sanyo Chemical's corprate website |
|---|
| UMEX 1001, UMEX 100TS, UMEX 1010, UMEX 110TS |


- What is a surfactant
- Surfactant functions introduction video
- What is a dispersant
- Paints and Dispersants
- Printing Inks and Dispersants
- Paper and Dispersants
- Cosmetics and Dispersants
- Cement and dispersants
- Pesticides and dispersants
- Dyeing and Dispersion
- Plastic coloring and dispersion
- Related Information
This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.