Surfactant Basics 3 (Penetrants, Wetting Agents, Fabric Additives)
What is a surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
Basic structure of a surfactant
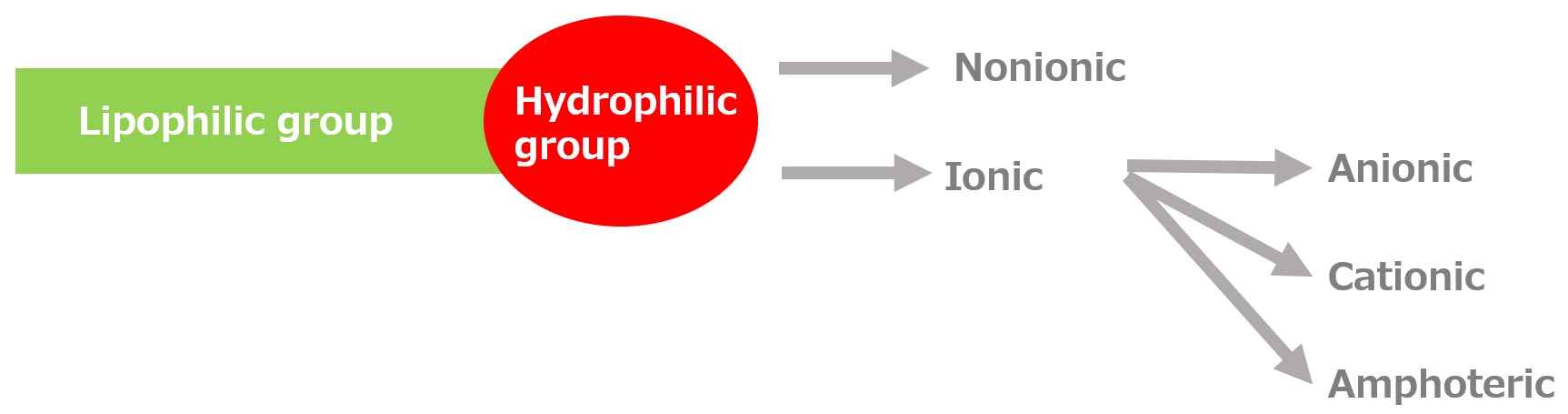
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-fitting parts) and hydrophilic groups (water-fitting parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant | -Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH | -Clothing detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil | -Polyoxyethylene alkyl ether etc. |
| Anionic surfactant | -Excellent emulsification and dispersibility -Good lather -Temperature insensitive | -Clothing Detergent -Shampoo -Body soap | -Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant | -Adsorption to fibers -Antistatic effect -Bactericidal | -Hair rinse -Fabric softener for clothes -Disinfectant | -Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant | -Mild on skin -Excellent solubility in water -Synergistic with other active components | -Shampoo -Body soap -Kitchen detergent | -Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant functions introduction video
It consists of seven short movies for each function.
0:00 Introduction of surfactants functions
0:16 Part1 Washability
1:00 Part2 Permeability
2:10 Part3 Dispersion
2:55 Part4 Foaming properties
3:25 Part5 Defoaming properties
3:39 Part6 Smoothness
4:20 Part7 Antibacterial properties
What are wetting agents and penetrants?
Wetting agents and penetrants are used to wet or soak up water. Wetting and penetrating are similar, and wettability is important. Surfactants with almost the same composition are used for wetting and penetrating agents.
Wetting: Replacing the interface between a solid and a gas with the interface between a solid and a liquid (water) → Wettability
Penetration: Liquid (water) enters the gap between solids → soaking in
Wetting Classification
Usually, wetting can be classified into three categories.
| Summary | Rough sketch | Example | |
|---|---|---|---|
| Spreading wetting | Droplets cover the entire surface of the solid surface | 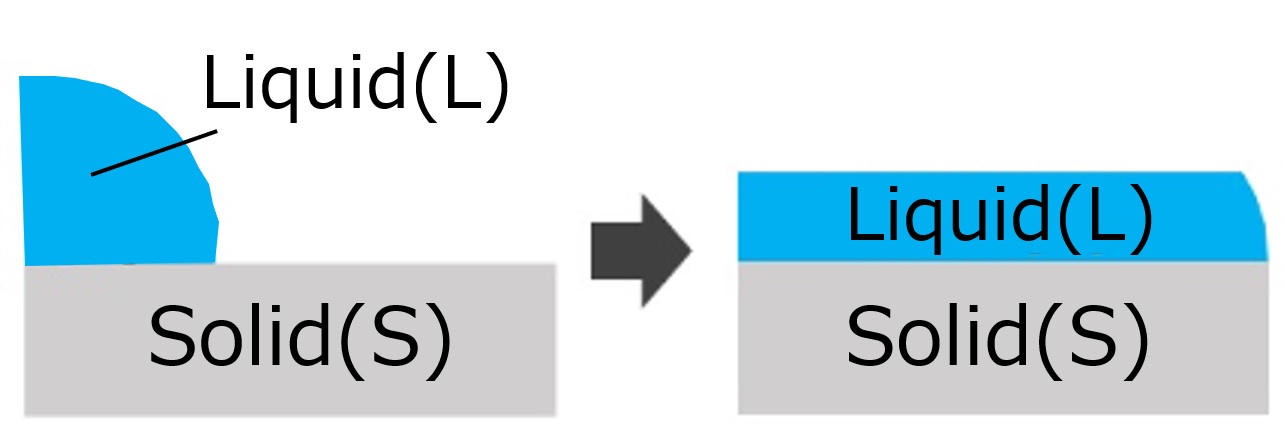 | ・Rain wetting the asphalt surface. ・Spreading over surface during coating |
| Adhesional wetting | Wet to place droplets on solid surfaces | 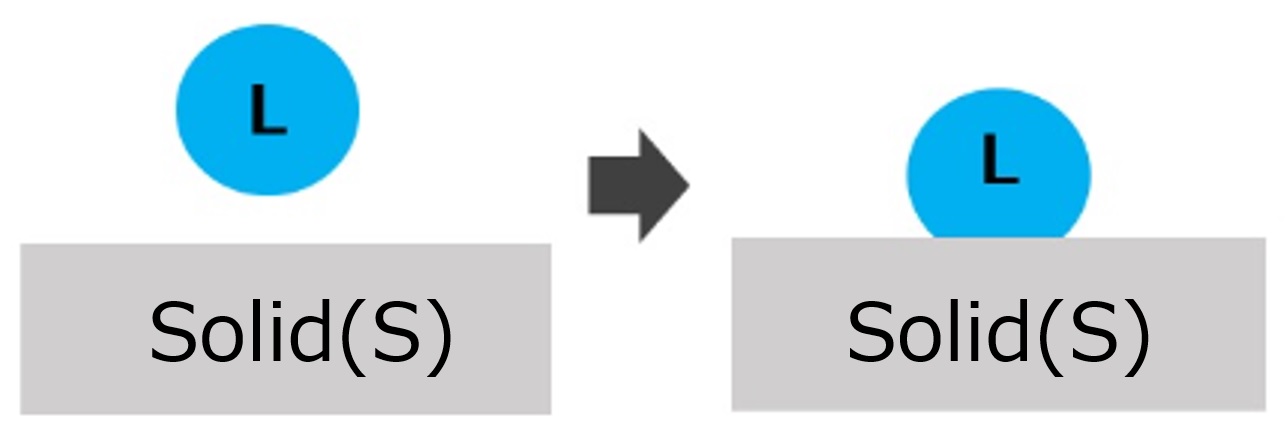 | ・Water droplets sticking to a leaf ・Steam fogging on glass surfaces ・Adhesive or ink application |
| Immersional wetting | Immersing solids in large volumes of water | 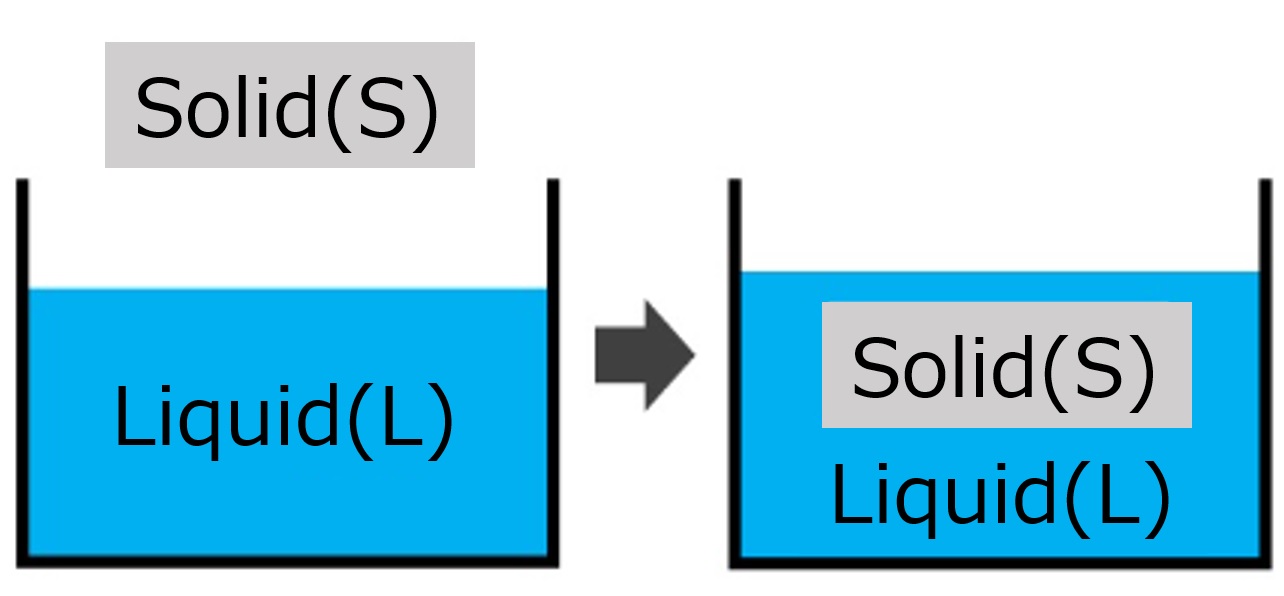 | ・Flour mixed with water ・Wetting of fibers ・Dispersion of pigments and powders |
Wettability index is related to contact angle
The shape of a water droplet is used to quantitatively express wettability. The shape of a water droplet can be expressed by the contact angle θ as shown in the figure below.
The closer the contact angle is to 0 degrees, the easier it is to wet, and the closer it is to 180 degrees, the harder it is to wet
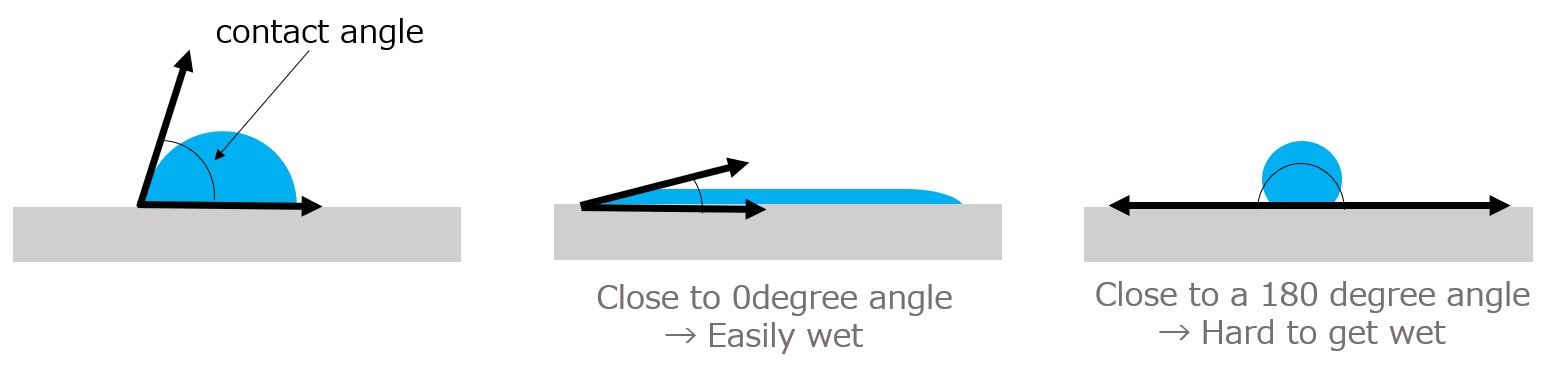
Fig. State of wetting of solid surface
When there is no surfactant
A droplet of water tries to become spherical with the smallest surface area due to the surface tension of the water (to minimize the surface energy of the droplet).
Also, the glass surface is stabilized by lowering its energy by adsorbing gas molecules on the surface.
⇒When a water droplet comes in contact with glass, the droplet becomes spherical on the glass (i.e., not wet)
When there is a surfactant
Surfactant collects at the contact point where solid and liquid repel each other, and water droplets spread easily.
⇒The contact angle of water droplets on the glass surface becomes smaller.
Relationship between contact angle and surface tension
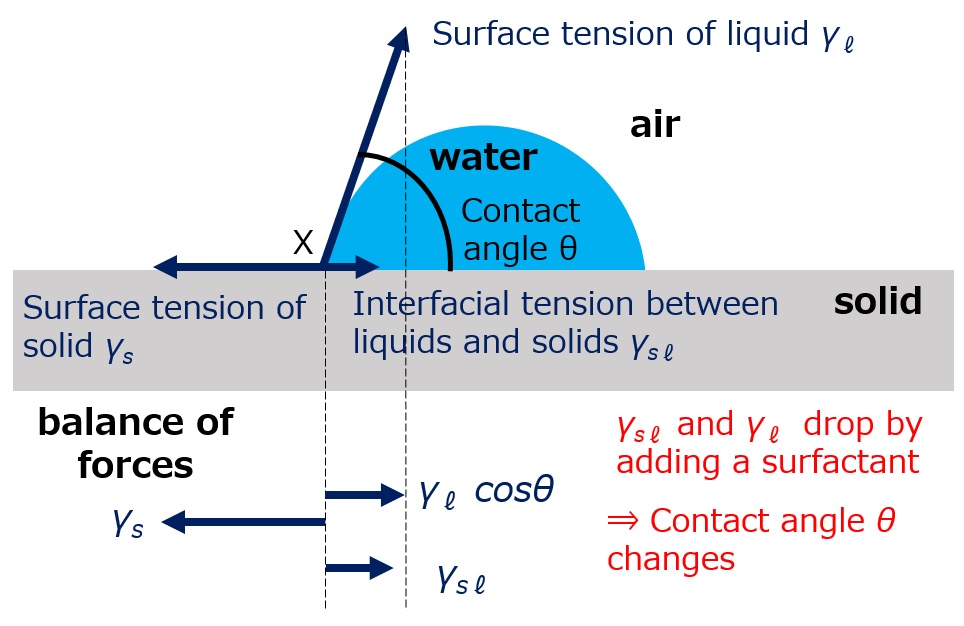
Fig. Solid surface wet with water droplets
The wetting of a solid surface by a water droplet is represented by three forces (liquid surface tension γℓ, solid surface tension γs, and liquid-solid interface tension γsℓ) and the contact angle θ, as shown in the left figure.
If these three forces are summed up and a water molecule is pulled further to the left at contact point x, the water droplet will spread more and more, wetting the solid surface and reducing the contact angle θ. If the water droplet is pulled to the right, it will spread more and more, wetting the solid surface. If it is pulled to the right, the opposite is true.
Force pulling to the left: γs
Force to pull to the right: γℓ-cosθ + γsℓ
By comparing the magnitude of these forces, we can express wettability.
If γs > γℓ⋅cosθ + γsℓ: water droplets tend to spread out
If γs < γℓ⋅cosθ + γsℓ: water droplets try to approach a spherical shape
If γs = γℓ⋅cosθ + γsℓ: the water droplet is stationary and θ at this time is the contact angle.
Further transforming this equation, the contact angle can be expressed in terms of surface tension and interfacial tension as follows.
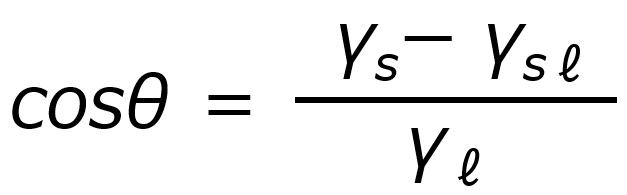
Mechanism of action of wetting and penetrating agents
Behavior of surfactant dissolved in a drop of water
Surface tension of solid γs: Constant for different types of solids (does not change with surfactant)
Surface tension of water γℓ: Decreases with the addition of surfactant
Surface tension between water and solid γsℓ: Decreases with addition of surfactant
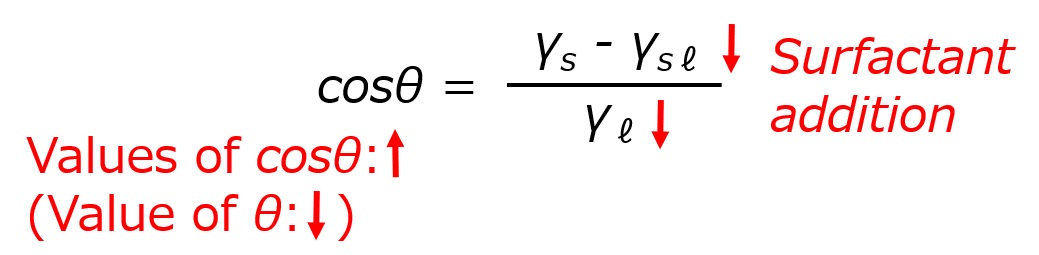
When a surfactant is added, the right-hand side of the above equation becomes a large number, and the contact angle θ on the left-hand side must become smaller to balance it. Thus, the surfactant's action of lowering the interfacial tension decreases the contact angle θ, which in turn increases the wetting and penetrating actions. This is the mechanism of action of wetting and penetrating agents.
Surfactants as wetting and penetrating agents
Penetration power of typical surfactants
| Surfactant | Penetration time s |
|---|---|
| Nonylphenol EO 10 mol adduct | 4.0 |
| sodium dodecyl benzene sulfonate | 3.0 |
| sodium alkylnaphthalene sulfonate | 4.5 |
| Sodium dioctyl sulfosuccinate | 1.0 |
| water only | >100 |
Measurement method: Canvas method (0.5% aqueous surfactant solution at 15°C)
EO 10 mol adduct in the table means ethylene oxide 10 mol adduct.
In plain water without a permeating agent, the floating canvas does not settle forever, but when a permeating agent is added, it settles in just a few seconds.
Among these, aqueous solutions to which sodium dioctyl sulfosuccinate is added exhibit particularly excellent permeability.
Penetrating power of various nonionic surfactants
| Non-ionic surfactant | Lipophilic group structure | Cloud point ℃ | Penetration time s | |
|---|---|---|---|---|
| 0.2% aq. | 0.05 aq. | |||
| Paraffin oxidized alcohol EO adduct | With OH near the middle of the molecule C12-14 secondary alcohols |
64 | 4 | 28 |
| Oxo alcohol EO adduct | Branched C12-13 primary alcohols | 65 | 16 | 53 |
| Cheeghler alcohol EO adduct | Linear C12-16 primary alcohols | 64 | 60 | 203 |
| Nonylphenol EO adduct | Alkylphenol with branched side chains | 63 | 4 | 24 |
| Linear alkylphenol EO adduct | Linear alkyl phenol | 56 | 5 | 27 |
In terms of wetting and penetration effects, surfactants with a hydrophilic group in the middle of the lipophilic group have the greatest penetration power. Surfactants with a linear lipophilic group and a hydrophilic group at the end have the least penetrating power.
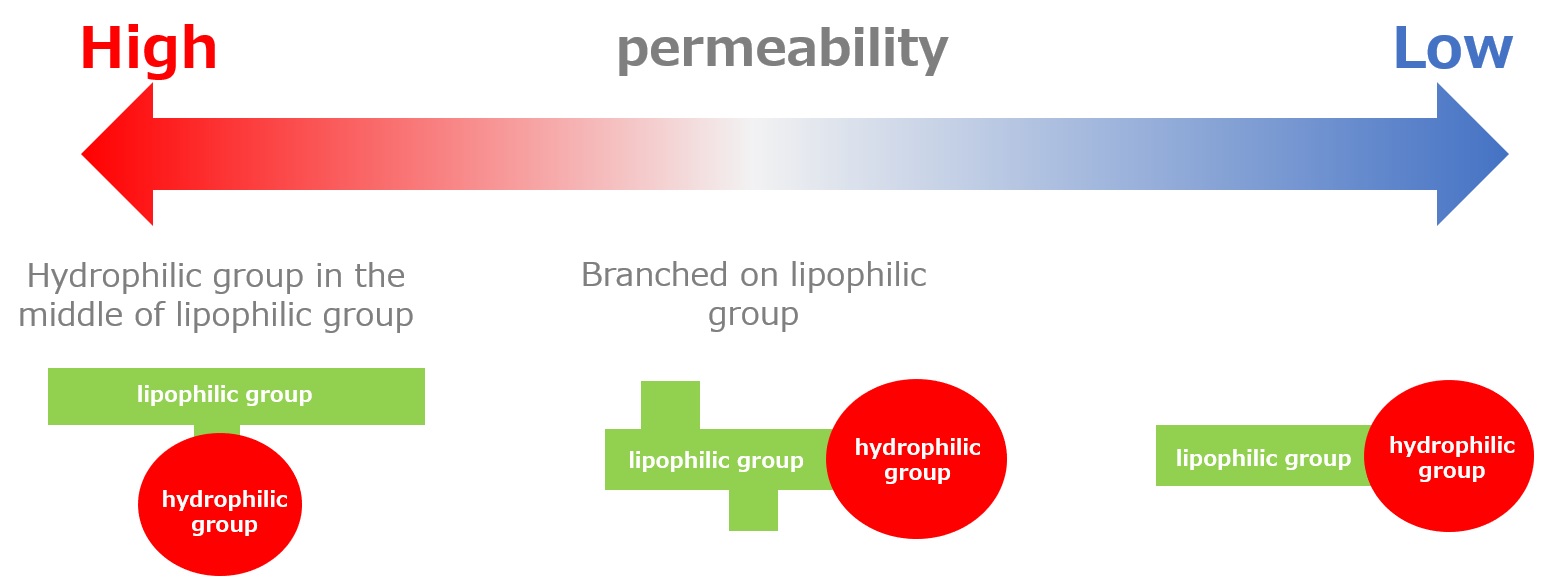
Fig. Comparison of Penetration Power of Higher Alcohol EO Adducts
This rule of thumb is well known to be generally valid not only for nonionic surfactants but also for anionic surfactants. However, it is based on the precondition that the surfactant is in a suitable range for wetting and penetration.
Wetting agents and penetrants applications
Textile industry

With the advancement of textile processing technology, penetrants and wetting agents have become indispensable agents in a variety of situations. They are used under a wide range of conditions, from highly acidic to highly alkaline, and in situations where large amounts of salt are present.
Fibers vary in wettability depending on their chemical structure. The table below lists fibers that are easily wettable and fibers that are not easily wettable.
In reality, fibers often contain oils or natural waxes, which may differ from this classification.
Classification of fiber wettability
| Very Wettable Fibers | Nonfat cotton, rayon, vinylon, acrylic |
|---|---|
| Relatively Wettable Fibers | Acetate, nylon |
| Slightly wet fibers | Polyester |
| Hardly wettable fibers | Polypropylene, wool |
Properties of treatment solutions containing wetting and penetrating agents
In the manufacturing process leading up to the production of cloth, the conditions under which the fibers are treated may be acidic or alkaline, or may contain large amounts of inorganic salts. These factors can also greatly affect the penetrating power of penetrants.
Examples of factors that reduce penetrating power
-In general, non-ionic penetrants often lose their penetrating power in alkaline conditions.
-Anionic penetrants often lose their penetrating power in acidic conditions.
-Penetrants with ester bonds in their molecules may decompose at high temperatures in strongly acidic or strongly alkaline environments, resulting in reduced penetrating power.
-Sulfate-type anionic penetrant ester salt penetrant may decompose in acidic solutions, resulting in a decrease in penetrating power during use.
Effect of acids, alkalis and salts on penetration time
| Penetration time [s] | ||||
|---|---|---|---|---|
| water | 3%NaOH | 5%H2SO4 | 10% NaCl | |
| C8~10 alcohol EO6 mol adduct | 3.5 | 10.3 | 10.1 | 2.5 |
| Nonylphenol EO 10 mol adduct | 4.0 | 37.0 | 3.0 | 57.4 |
| Sodium alkylnaphthalene sulfonate | 4.5 | >60 | insoluble | insoluble |
| Sodium dioctyl sulfosuccinate | 1.0 | 3.0 | 3.5 | insoluble |
Conditions of use and an example of a suitable penetrant
| Conditions of use | Penetrant used |
|---|---|
| Strongly alkaline | Alkaline penetrants, mainly low molecular weight anionic penetrants |
| Weak alkalinity | Anionic penetrant such as sulfate ester salt type, non-ionic penetrants, etc. |
| Slight acidity | Sulfonate-type anionic penetrant, non-ionic penetrant |
| Strong acidity | non-ionic penetrants |
| Solution with high concentration of strong oxidizing agents or inorganic salts | Special sulfonate-type penetrants Example Din sodium alkyl diphenyl ether disulfonate |
Wetting and penetrating agents in the synthetic fiber manufacturing process
Synthetic Fiber Manufacturing Process Flow
Polymer production ⇒ Spinning ⇒ Drawing ⇒ Post-treatment process
Role of fiber oil
Fiber oil mainly refers to a spinning oil agent that facilitates yarn pulling in the prevention process. Penetrating and wetting agents are added to help the agent work effectively.
Effects of Wetting and Penetrating Agents on Polypropylene Fibers
The following is an example of a fiber oil treatment process for polypropylene fiber, which is the most difficult to wet with water.
When treating polypropylene fibers with oil, the oil is emulsified or dispersed in water.
Polypropylene fibers are not easily wetted by water, which can cause problems such as "oil does not adhere easily" and "oil does not penetrate into the fiber bundle.To solve this problem, non-ionic or anionic wetting/penetrating agents are usually added to the oil to provide wetting/penetrating action.
Higher alcohol EO adducts and sulfosuccinic acid type surfactants, which have a low contact angle and excellent penetrating power, are added to make it easier for the oil to penetrate into the polypropylene fiber bundles.
Effects of Wetting and Penetrating Agents on Polypropylene Fibers
| Wetting and penetrating agents | Contact angle [°] 0.5% solution |
Penetration time [s] 1.0% solution |
|---|---|---|
| None (water) | 78 | not settle |
| Higher alcohol EO adduct | 28 | 2 |
| Sulfosuccinic acid type | 30 | 6 |
| Oil A (no wetting/penetrating agent added) | 68 | Approx. 200 |
| Oil A + 10% of the above higher alcohol EO adduct added to the oil | 63 | 13 |
Wetting and penetrating agents for refining processes
Before dyeing, natural and synthetic fibers alike are coated with oil and glue during the spinning and weaving processes, as well as with dust and machine oil. These deposits can cause a variety of problems during textile processing.(For example, they prevent dye penetration and cause uneven dyeing.)
In order to prevent these problems from occurring, the dyeing process involves a process called refining, which completely removes these adhering substances before dyeing.
Major impurities present in the fabric before the fiber is dyed
| Type of stain | |
|---|---|
| For natural fibers | Solids, fats, oils, proteins, pectic substances, mineral oils, saponification products of fats and oils, pigmented substances |
| Process-derived impurities | Spinning oil, glue, machine oil, dirt such as iron rust, cotton waste, etc. |
Examples of penetrants used in cotton refining
Cotton is very resistant to alkali, so it is often refined using a combination of strong alkali such as sodium hydroxide or sodium carbonate and anionic or nonionic penetrants. Cotton is also resistant to heat, and is refined at high temperatures to achieve the refining effect in a short time.
Effect of Penetrant on Cotton Cloth Refining
| Refining bath composition | ||||
|---|---|---|---|---|
| A | B | C | D | |
| NaOH(38° Boehme) | 10 | 10 | 10 | 10 |
| Oleyl alcohol sulfate sodium salt* | ー | 1 | ー | 1 |
| Oleyl alcohol EO18 mol adduct | ー | ー | 0.2 | ー |
| highly sulfated oil | ー | ー | ー | 1 |
| water | 90 | 89 | 89.8 | 88 |
| Whiteness of refined cloth** (%) | 70.5 | 72.7 | 75.3 | 74.2 |
Wetting and penetrating agent for gluing process
In the weaving process, glue is applied to the warp threads.
Starch and gelatin used to be used as glue, but sodium alginate, carboxymethyl cellulose (CMC), polyvinyl alcohol (PVA), and other synthetic glues are now being used.
The greater the amount of glue adhered to the warp, the more efficient the gluing process. Penetrants are used to increase the penetration of the glue, allowing more glue to adhere to the fibers in a shorter period of time.
Relationship between glue penetration time and adhesion amount
| Penetration time of glue [sec] | Amount of glue adhered to warp yarn % |
|---|---|
| 600 or more | 2.7 |
| 310 | 3.0 |
| 8 | 3.2 |
The table on the left shows the relationship between glue penetration time and glue adhesion. The shorter the glue penetration time, the greater the amount of glue adhered.
-Warp yarn type: Cupra fiber
-Glue solution composition: PVA 3%, weaving oil (containing a non-ionic penetrant) 0.3%, and water 96.7%,
-Gluing method: Small roller sizing machine
-Measurement method: Canvas method (20°C)
Wetting and penetrating agent for bleaching process
Penetratants are used to enhance the effectiveness of bleaching agents in whitening cloth.
Bleaching Method Classification
| Fluorescent bleaching method | A method to improve whiteness by having fibers absorb a fluorescent substance that absorbs ultraviolet rays and emits short-wavelength light rays in the visible. This method is easy to use and has an excellent bleaching effect, but the degree of whiteness appears to change markedly depending on the light source, however, the degree of whiteness appears to change markedly depending on the light source, and the degree of sunlight hardness is poor. |
|---|---|
| Chemical bleaching | A method to decompose pigment substances contained in fibers by oxidative or reducing action to produce pure white. |
As bleaching methods become more mechanized, becoming a continuous process and faster in speed, it is necessary to ensure uniform penetration of the bleaching solution in a short time.
Therefore, the superiority of the penetrant becomes the deciding factor. In addition to this, low foaming is also important in continuous production, in addition to permeability.
Bleaching of cotton and rayon fabrics is often done with sodium chlorite in a weak acidic solution.
Nonionic penetrants are suitable or blends with anionic activators are used.
Wetting and penetrating agents in the dyeing process

Wetting and penetrating agents are added to the dyeing solution in order to make the dye penetrate quickly into the fiber bundles and to dye them evenly.
Classification of Dyeing Methods
Dip-dyeing: Dyeing is performed by immersing the fiber in a dye bath (Jigger dyeing machines, Winth dyeing machines, liquid flow dyeing machines).
Textile dyeing: A dyeing method in which dye is added to a glue agent, a printed pattern is stamped on the cloth to create a multi-colored pattern, and then heated with steam to dye the dye onto the fibers.
In the case of natural fibers such as cotton and hemp, and recycled fibers such as viscose rayon, ionic groups do not exist on the surface of these fibers as adsorption sites, so direct dyes, naphthol dyes, sulfide dyes, building dyes, and reactive dyes are used as dyes to be used.
The first stage of dyeing is the penetration of dye solution into fibers, and how well it penetrates at this stage greatly affects the uniformity of dyeing. For this reason, emphasis is placed on the penetration function of the dyeing auxiliaries used.
The following are examples of penetrating agents used in dyeing.
Sodium dioctyl sulfosuccinate
Sodium alkylbenzenesulfonate
Sodium alkyl sulfates
Higher alcohol EO adduct
Wetting and penetrating agent for mercerization process
Mercerization
When cotton fibers are immersed in a 20-30% dark sodium hydroxide solution near room temperature, the cotton fibers swell and thicken due to the alkali and at the same time begin to shrink rapidly in length. At this time, if the fibers are pulled to prevent shrinkage, they become lustrous and easily dyeable cotton fibers.
Cotton golf shirts and blouses with a silk-like luster are made using this method.
Uniform and rapid penetration of the alkali solution into the cotton fabric is also important in the mercerization process, but a special penetrant is required because ordinary penetrants do not dissolve in such a thick alkali solution at all.
Alkali Penetrant
As shown in the table below, surfactants with small hydrophobic groups are suitable as alkali penetrating agents. Small hydrophobic groups are too soluble in ordinary water to show much surfactant activity, but in concentrated alkaline solutions, their solubility decreases to an appropriate level, resulting in excellent penetrating power.
Comparison of alkaline penetrant and ordinary penetrant
| Alkali penetrant | Normal penetrant |
|---|---|
small hydrophobic group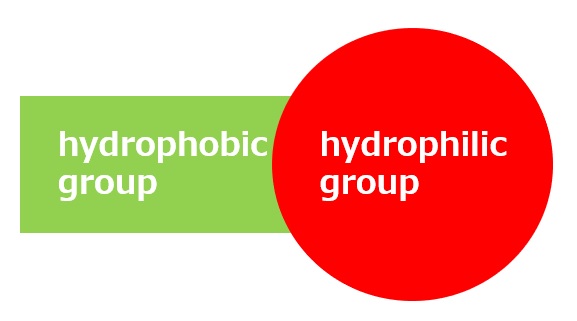 | large hydrophobic group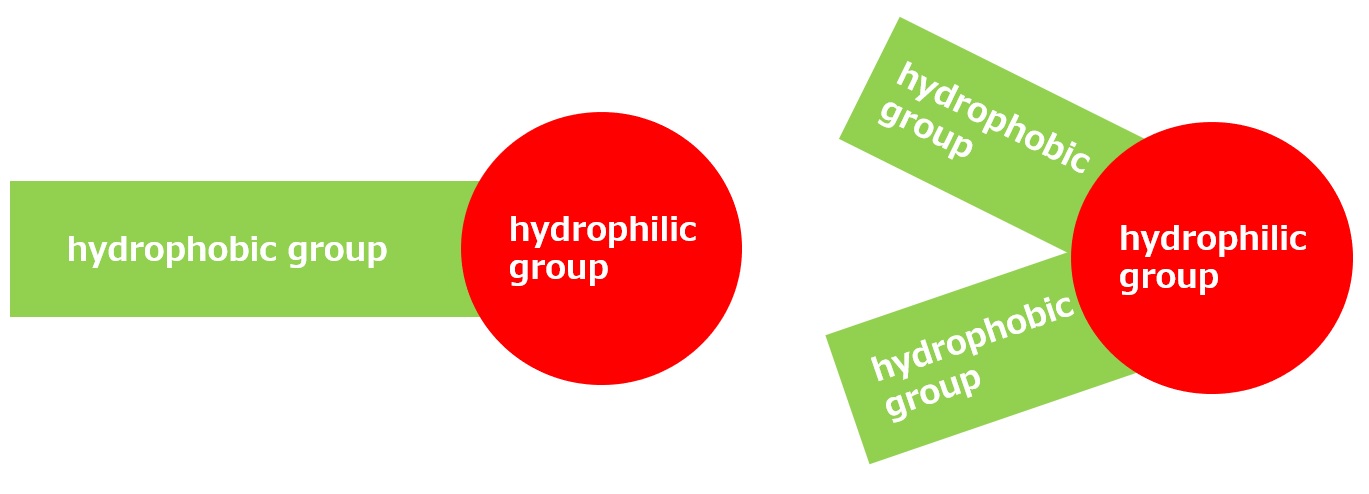 |
| Soluble in concentrated alkalis, but has low permeability in neutral water | Insoluble in concentrated alkalis |
Nowadays, anionic penetrants such as sulfonates and sulfates with lower alkyl groups of about 5 to 10 carbons are used in combination with solvents such as butyl cellosolve.
R(C5-C10)-SO3Na
R(C5-C10)-OSO3Na
C4H9OCH2CH2OH (butyl cellosolve)
Toiletries
Clothing detergents, kitchen detergents and shampoos

Soap, detergent, and shampoo, all everyday household products, have surfactants as their main ingredient, and their purpose is the same: to remove dirt and stains.
Cleaning is achieved when the various functions of surfactants, such as wetting, penetration, emulsification, and dispersion, are combined.
Example of washing process of clothes
1) Process of penetration of detergent solution into the crevices of fibers
2a) The process of separating stains from the surface of fibers
2b) The process of dispersing and protecting the stain
3) The process of removing stain from the cleaning system
Of these three processes, process (1) is to wet the fibers and allow the detergent solution to penetrate into the gaps between the fibers and make contact with the stains in order to remove the stains, which is almost unnecessary when cleaning smooth solid surfaces such as tableware and metals.
Next, the penetrating cleaning solution pulls the stain away from the fibers in the process of 2a).
In process (2b), the detached dirt particles are dispersed into smaller particles, and the emulsifying, dispersing, and anti-re-agglomeration action of the detergent is responsible for protecting the dirt particles from re-agglomeration once they are dispersed.
γow:Interfacial tension between cleaning solution and oil stain,
γws:Interfacial tension between cleaning solution and fiber,
γos:Interfacial tension between fiber and oil stain,
θ:Contact angle
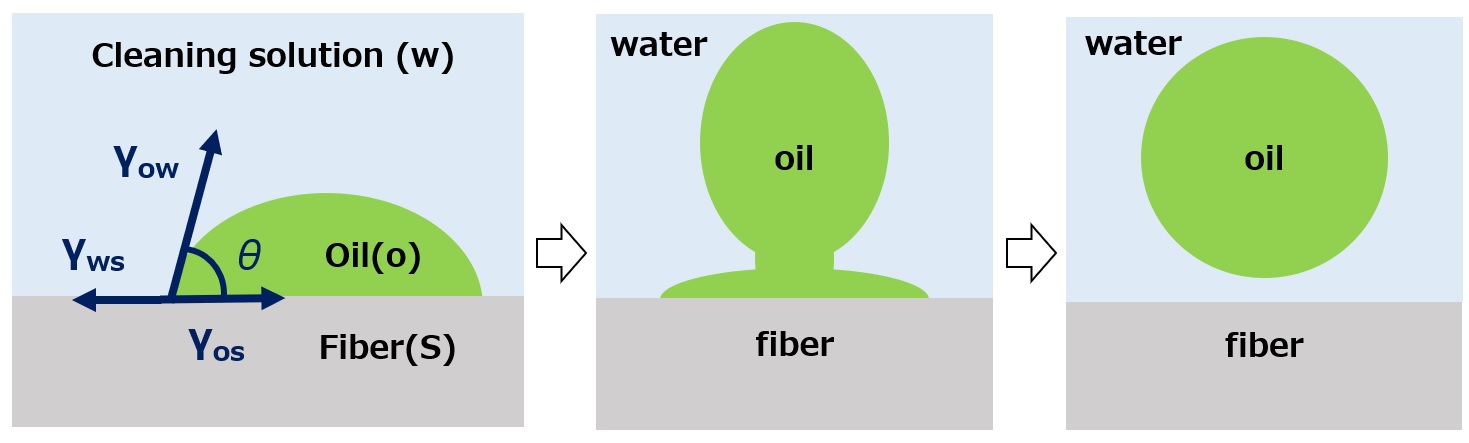
Fig. Concept of washing mechanism
Surfactants used in laundry and kitchen detergents and shampoos
| Type | Typical surfactants used |
|---|---|
| Clothing Detergent | Anionic surfactants such as sodium dodecylbenzenesulfonate |
| Kitchen detergent | Anionic surfactants such as EO adducts of higher alcohols, sodium alkyl ether sulfates |
| Shampoo | Anionic surfactants such as sodium alkyl ether sulfates |
For more information on cleaning, please also see the following pages.
Surfactants Basics 1 (Detergent)
Mold Remover Detergent

Mold remover detergent is often used in bathrooms during general cleaning and the rainy season.
Mold on tile joints and ceilings is not easily removed by simply washing with a mild detergent or scrubbing with a cleanser.
For this reason, the most commonly used method is to use an oxidizing agent such as sodium hypochlorite or hydrogen peroxide, which uses its oxidizing power to kill the mold and decompose and bleach the mold pigment to make it colorless.
Although it is possible to dissolve and use surfactants such as penetrating agents and detergents when using an oxidant, the problem of oxidative degradation of surfactants during storage arises when an oxidant and surfactant are used together. Therefore, it is necessary to select a surfactant that does not have easily oxidizable bonds.
When using hydrogen peroxide
Nonionic surfactants such as higher alcohol EO adducts can be stably blended.
When using sodium hypochlorite
Alkalinity is required to keep sodium hypochlorite stable.
⇒ Must be stable in oxidative stability and alkalinity.
Since higher alcohol EO adducts are oxidatively degraded, sulfonic acid type anionic surfactants are usually used.
Surfactants used in mold remediation detergents
| Surfactants used | structural formula |
|---|---|
| Sodium alkyl diphenyl ether disulfonate | 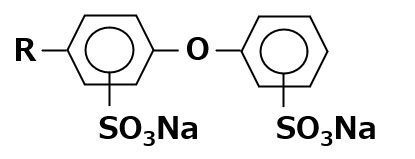 |
| Sodium polyoxyethylene alkyl phenyl ether sulfate | 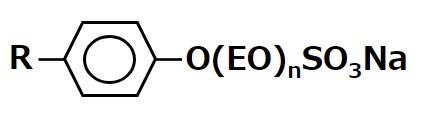 |
| Sodium alkane sulfonate | RSO3Na |
| Saturated fatty acid soap | RCOONa |
Agriculture
Agricultural Spreading Agents

Direct wetting of plants and insects is important for pesticides to be effective even in small amounts. Surfactants are also used in pesticides to provide this wetting property.
Main forms of pesticides used
(1) Emulsion: Liquid form, diluted with water and sprayed in the form of an emulsion.
(2) Hydrate: In a fine powder form, it is dispersed in water before use and sprayed.
(3) Powder: Sprayed as it is in fine powder form.
Surfactants used as Spreading Agents for Agriculture
Among the above, surfactants are mainly used as wetting agents in emulsions and hydrates, as shown in the table below. Surfactants derived from silicones have superior surface tension lowering ability compared to hydrocarbon-based surfactants.
Usually, the use of 0.01~0.1% of wetting agent as a spreading agent for agriculture, above the critical micelle concentration (c.m.c.), is sufficient in cases such as tridecyl alcohol EO adduct, but in many cases, plants are targeted, and sufficient attention should be paid to chemical damage to plants.
Surfactants used as agricultural spreaders
| Surfactants used | structural formula |
|---|---|
| Isotridecyl alcohol EO adduct | iso-C13H27O(EO)nH |
| Sodium dodecyl benzene sulfonate | 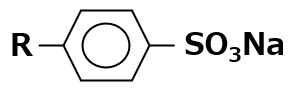 |
| Sodium dioctyl sulfosuccinate | 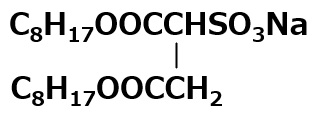 |
| Polyether-Modified Silicone |
Golf course turf dry spot preventer
Dry spot is the term for turf dieback that occurs on golf courses during hot weather. Anaerobic bacteria present in the roots of the turf produce a waxy substance through metabolism, which creates an impermeable layer near the soil surface, making it difficult for rainwater or sprinkled water to penetrate into the soil, which is then heated by the sun, resulting in a waterlogged condition.
The surfactant used in this process acts as an infiltrating agent that facilitates water penetration and emulsifies a waxy substance to prevent the formation of an impermeable layer. Therefore, as with agricultural spreading agents, the required functions of dry spot inhibitors are excellent surface lowering ability and low chemical damage.
As with spreading agents, the amount of surfactant used as a dry spot inhibitor should be sufficient as long as the concentration is above the critical micelle concentration, and the amount and frequency of application should also be carefully considered to prevent chemical damage.
Surfactants used as dry spot inhibitors
| Surfactants used | structural formula |
|---|---|
| Higher alcohol EO adduct | RO(EO)nH |
| Higher fatty acid sorbitan esters | 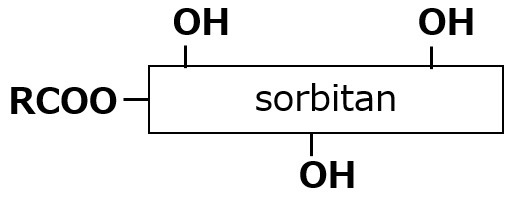 |
| Polyether-modified silicone |
Plastics & Inks

Various pigments (inorganic and organic) are used in paints, inks, and plastics for coloring and other purposes. If these pigments clump together or are not uniformly dispersed, vivid colors will not be produced. To solve this problem, agents called dispersants are used.
Wetting and osmosis play an important role in the function of these dispersants. For solid particles to disperse well in a liquid, one of the prerequisites is that the surface of the particles be well wetted by the liquid.
For example, pigments such as carbon black are not wettable in water, so they float on the surface of water and are difficult to disperse in water. Thus, wetting is a factor that has a great influence on the dispersion system.
Water-based emulsion paint
Aqueous emulsion paints consist mainly of resin emulsions and inorganic pigments, to which are added dispersants and other additives.
Inorganic pigments are made up of agglomerates (secondary particles) consisting of several to several dozen fine particles (primary particles) ranging from 0.01 to several μm in diameter.
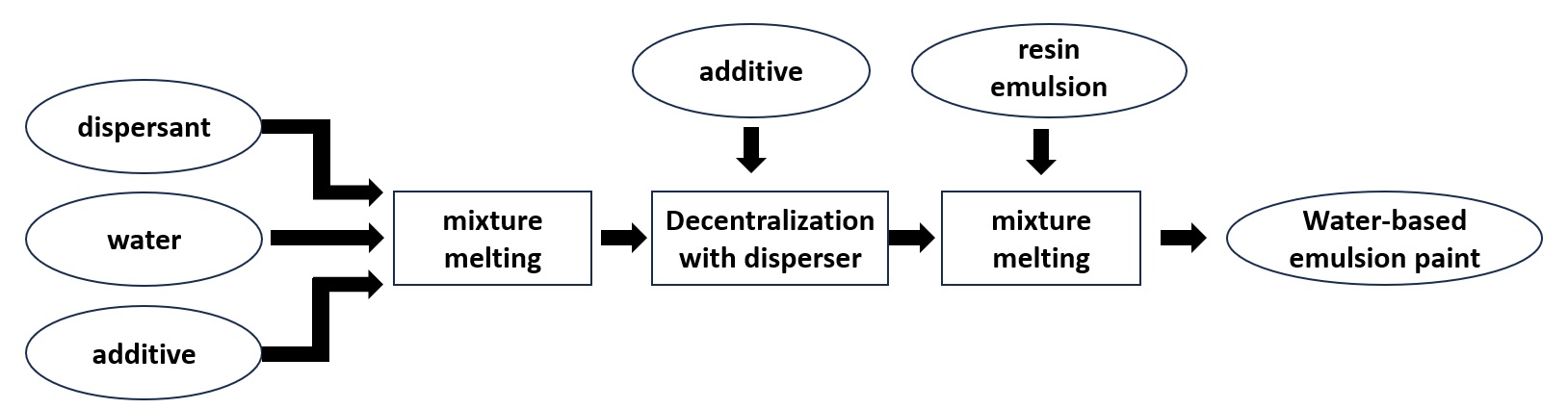
The dispersion solution in which the pigments have been dispersed shows vivid colors because the pigments are dispersed as fine particles. The paint is then made by adding a resin emulsion and a small amount of additives.
If no dispersant is added at all...?
(1) Dispersion efficiency by machine is reduced (dispersion takes a long time or requires strong physical dispersion force). If the dispersant alone is difficult to wet, a penetrant may be added separately.
(2) The color of the paint becomes dull due to reagglomeration of dispersed fine particles. Also, since agglomerated particles tend to settle, problems such as separation of the paint are more likely to occur.
Aqueous dispersants
Higher alcohol EO adduct
Sorbitan fatty acid esters
Sodium dioctyl sulfosuccinate
Formalin condensate of sodium naphthalene sulfonate
Sodium polystyrene sulfonate
Sodium polyacrylate
Carboxymethyl cellulose
Nonaqueous dispersants
Polyacrylic acid partial alkyl esters
Polyalkylene polyamine
Sanyo Chemical's surfactants suitable for wetting and imparting permeability
SANMORIN OT-70 (an anionic surfactant with excellent wetting and penetrating power)

- Anionic surfactant
Sulfosuccinate-type anionic surfactant (sodium dioctyl sulfosuccinate) "SANMORIN OT-70"
NAROACTY ID products (linear alcohol surfactant with improved penetration)
In general, linear alcohol surfactants have excellent emulsifying and dispersing power, but poor penetrating power.
Our proprietary ethylene oxide addition technology enables us to narrow the molar distribution of addition and synthesize surfactants with the targeted balance of hydrophilic and lipophilic properties, thereby increasing the penetration power of linear alcohol type surfactants.
For the Narrowacty ID series product introduction page (link to corporate website)
NAROACTY ID-40
NAROACTY ID-60
NAROACTY ID-70
Related Information
Surfactants & Textiles Product Introduction Page


| Link to product information on Sanyo Chemical's corporate website |
|---|
| Sodium Dioctyl Sulfosuccinate "SANMORIN OT-70" Non-ionic penetrant that provides wetting and penetrating properties in aqueous solutions of acids, alkalis, and salts "SANMORIN 11" "SANNONIC SS" products of polyoxyethylene (12-14) alkyl ethers with strong wetting and penetrating power SANNONIC SS-30, SANNONIC SS-50, SANNONIC SS-70, SANNONIC SS-90, SANNONIC SS-120 Sorbitan fatty acid esters with strong wetting and penetrating power "Ionet S" products IONET S-20, IONET S-60V, IONET S-80, IONET S-80S, IONET S-85 Polyoxyethylene sorbitan fatty acid esters with strong wetting and penetrating power "Ionet T" products IONET T-20C, IONET T-60V, IONET T-80V |
Topics

References
Performance Chemicals Function Series No.2 Wetting and Soaking: Wetting and Osmosis Functions
This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.





