Introduction to Nonionic Surfactant
What is a surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
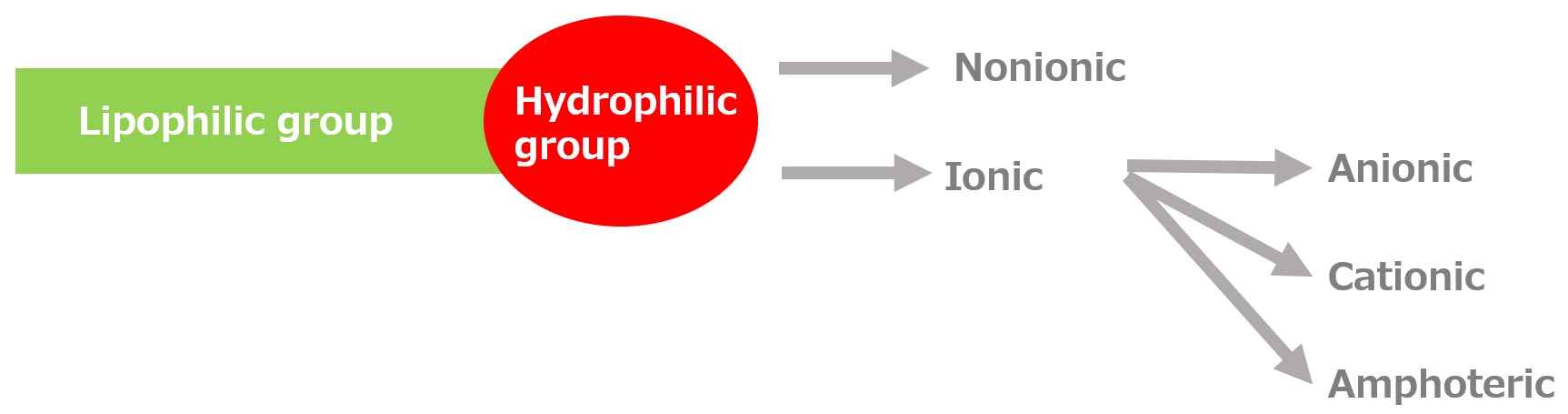
Basic structure of a surfactant
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-loving parts) and hydrophilic groups (water-loving parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant |
-Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH |
-Laundry detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil |
-Polyoxyethylene alkyl ether etc. |
| Anionic surfactant |
-Excellent emulsification and dispersibility -Good lather -Temperature insensitive |
-Laundry Detergent -Shampoo -Body soap |
-Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant |
-Adsorption to fibers -Antistatic effect -Bactericidal |
-Hair rinse -Fabric softener for laundry -Disinfectant |
-Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant |
-Mild on skin -Excellent solubility in water -Synergistic with other active components |
-Shampoo -Body soap -Kitchen detergent |
-Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant functions introduction video
It consists of seven short movies for each function.
0:00 Introduction of surfactants functions
0:16 Part1 Washability
1:00 Part2 Permeability
2:10 Part3 Dispersion
2:55 Part4 Foaming properties
3:25 Part5 Defoaming properties
3:39 Part6 Smoothness
4:20 Part7 Antibacterial properties
What is a nonionic surfactant?
Nonionic surfactants are used in the largest quantities among surfactants, and their raw materials, such as ethylene oxide, are stably supplied in large quantities.
Nonionic surfactants are surfactants that have hydroxyl groups (-OH) or ether bonds (-O-) as hydrophilic groups that do not dissociate into ions in water.
However, since hydroxyl groups and ether bonds do not dissociate into ions in water, their hydrophilicity is quite weak, so they alone do not have the power to dissolve large hydrophobic groups in water. Therefore, several of these groups come together in one molecule to exhibit good hydrophilicity. This is very different from anionic and cationic surfactants, where only one hydrophilic group is sufficient to exhibit hydrophilicity.
Classification of Nonionic Surfactants
Nonionic surfactants can be classified by the type of hydrophilic group into polyethylene glycol type and polyhydric alcohol type.
Polyethylene glycol-type nonionic surfactants are nonionic surfactants made by adding ethylene oxide as a hydrophilic group to a hydrophobic raw material.
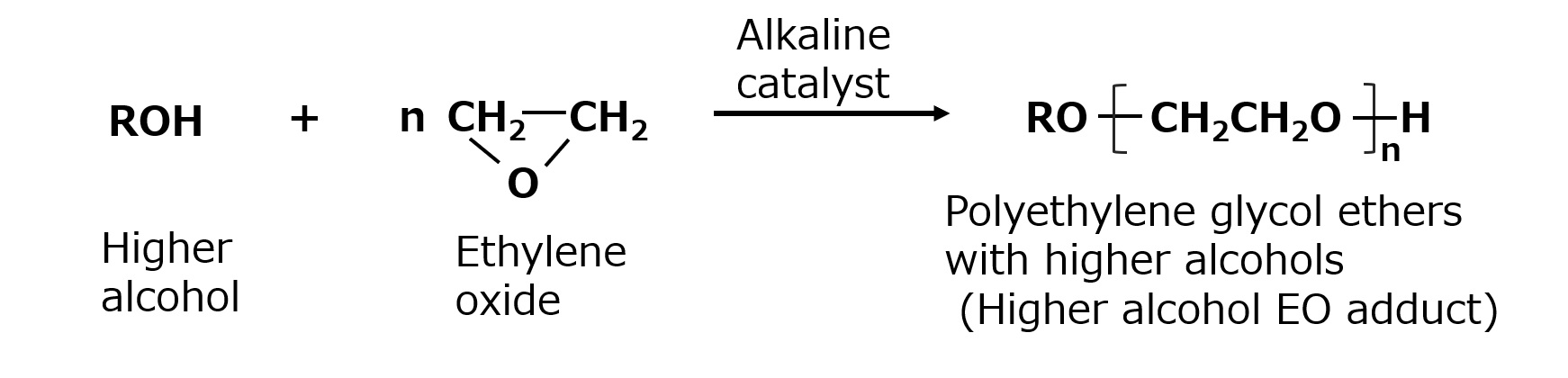
Fig. Example of polyethylene glycol-type nonionic surfactant
Polyhydric alcohol-type nonionic surfactants are nonionic surfactants in which a hydrophobic group such as a higher fatty acid is bonded to a polyhydric alcohol such as glycerol, pentaerythritol, sorbitol, etc. The hydrophobic group has multiple hydroxyl groups bonded to it, which gives it hydrophilic properties.

Fig. Example of polyhydric alcohol-type nonionic surfactant
A more detailed classification of polyethylene glycol-type nonionic surfactants is made according to the type of hydrophobic group, whereas in the case of polyhydric alcohol-type nonionic surfactants, it is made according to the type of polyhydric alcohol, the hydrophilic group. This classification is shown in the table below.
Classification of Nonionic Surfactants
| Polyethylene glycol type | Polyoxyethylene alkyl ether (Higher alcohol EO adduct) |
| Polyoxyethylene alkyl phenyl ether (alkyl phenol EO adduct) | |
| Polyoxyethylene fatty acid esters (Fatty acid EO adducts, polyethylene glycol fatty acid esters) |
|
| Polyoxyethylene polyhydric alcohol fatty acid esters (Polyhydric alcohol fatty acid ester EO adduct) |
|
| Polyoxyethylene alkylamine (Higher alkylamine EO adduct) | |
| Polyoxyethylene fatty acid amide (fatty acid amide EO adduct) | |
| Polyoxyethylene polyoxypropylene glycol (Polypropylene glycol EO adduct) |
|
| Other | |
| Polyalcoholic | glycerol fatty acid ester |
| Pentaerythritol fatty acid esters | |
| Sorbitol and sorbitan fatty acid esters | |
| Disaccharide fatty acid ester | |
| alkyl polyglycoside | |
| Fatty acid alkanolamide | |
| Other |
Polyethylene glycol type nonionic surfactant
Polyethylene glycol-type nonionic surfactants are surfactants made by adding ethylene oxide (EO: ethylene oxide) to hydrophobic raw materials containing reactive hydrogen atoms.
Reactive hydrogen atoms are specifically hydrogen atoms such as hydroxyl group (-OH), carboxyl group (-COOH), amino group (-NH2), or amide group (-CONH2). Hydrophobic groups bonded to the above atomic groups can react with ethylene oxide to form polyethylene glycol-type nonionic surfactants. For example, a higher alcohol with a hydroxyl group reacts to an EO adduct as follows
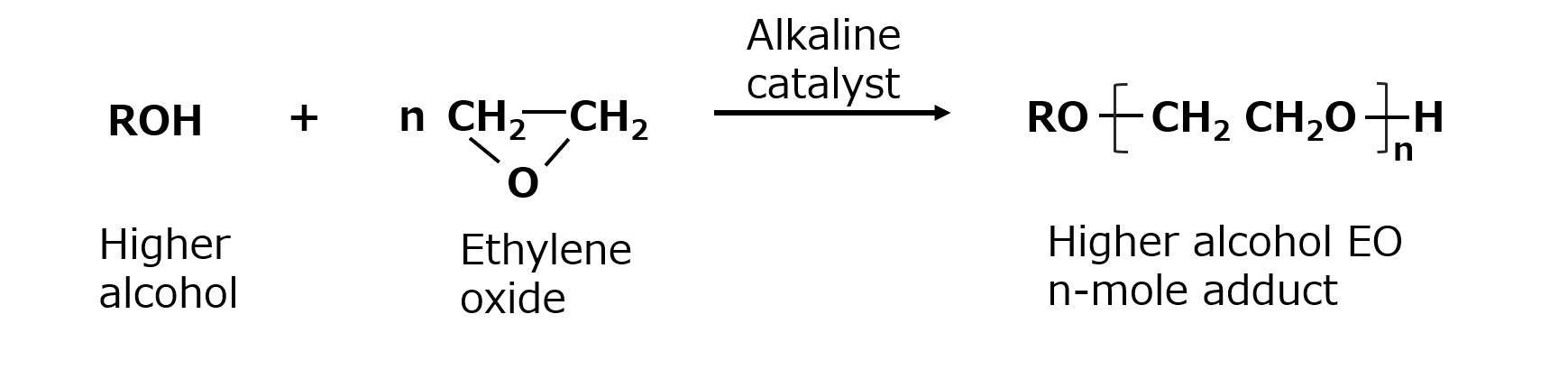
Fig. Reaction of higher alcohols with ethylene oxide
The following hydrophobic raw materials with reactive hydrogen atoms are currently used in relatively large quantities. Among these, higher alcohols are particularly important. However, alkylphenols are no longer used since it was found that they have endocrine disrupting effects.
Hydrophobic raw materials with easily reactive hydrogen atoms
| Classification of hydrophobic raw materials | Compound Examples |
|---|---|
| Higher alcohol: R-OH | Lauryl alcohol: C12H25-OH |
Alkyl phenols: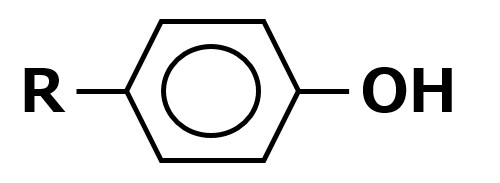 |
Nonylphenol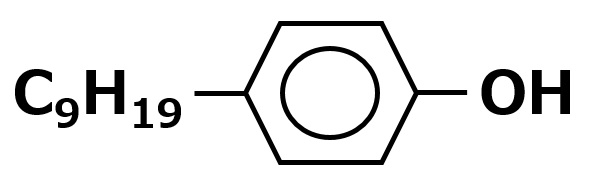 |
| Higher fatty acids: R-COOH | Oleic acid: C17H33-COOH |
| Higher alkylamine: R-NH2 | Stearylamine: C18H37-NH2 |
| Higher fatty acid amide: R-CONH2 | Oleic acid amide: C17H33-CONH2 |
Hydrophilicity and Cloud Point of Polyethylene Glycol Type Nonionic Surfactants
Hydrogen bonding and hydrophilicity of polyethylene glycol-type nonionic surfactants
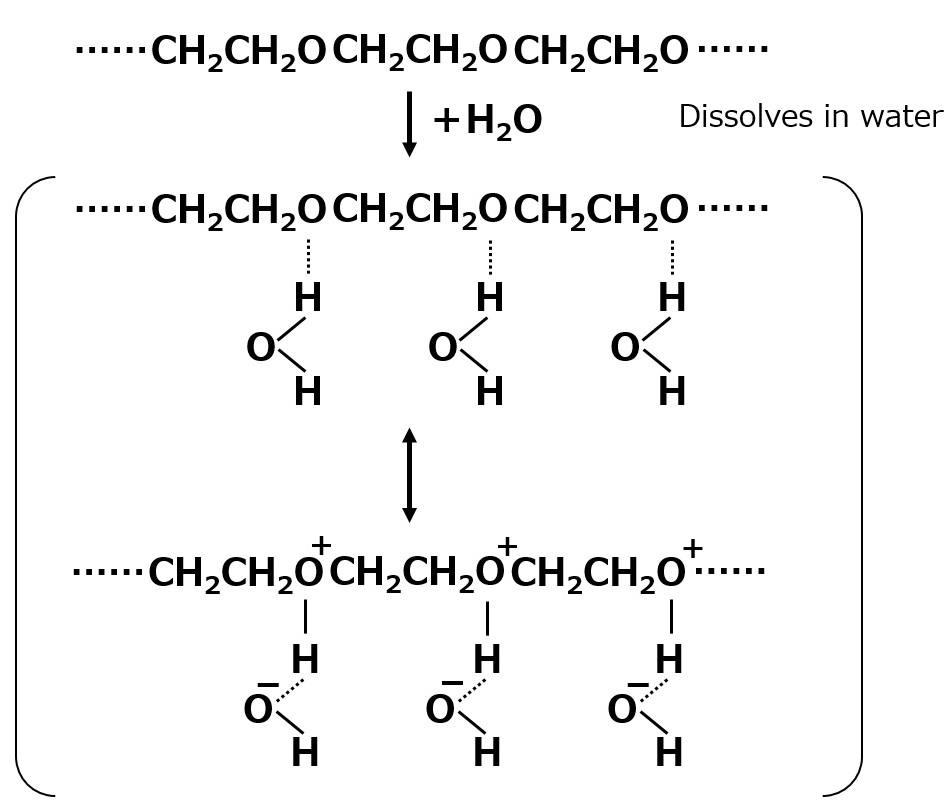
Fig. Hydrogen bond between polyethylene glycol chain and water molecule (conceptual diagram)
Polyethylene glycol chains are hydrophilic because water bonds loosely to the oxygen atoms of the ether bonds in the chain.
When an ether bond is hydrogen bonded to a water molecule, the surrounding water molecules see it as a peer, making it easier to dissolve in water. This is why they are hydrophilic.
In aqueous solutions of polyethylene glycol-type nonionic surfactants, water molecules are loosely attached to the ether bond sites by hydrogen bonds. Therefore, as the temperature rises or salts dissolve into the solution, the hydrogen bonds with the water molecules tend to gradually break off.
What is a cloud point?
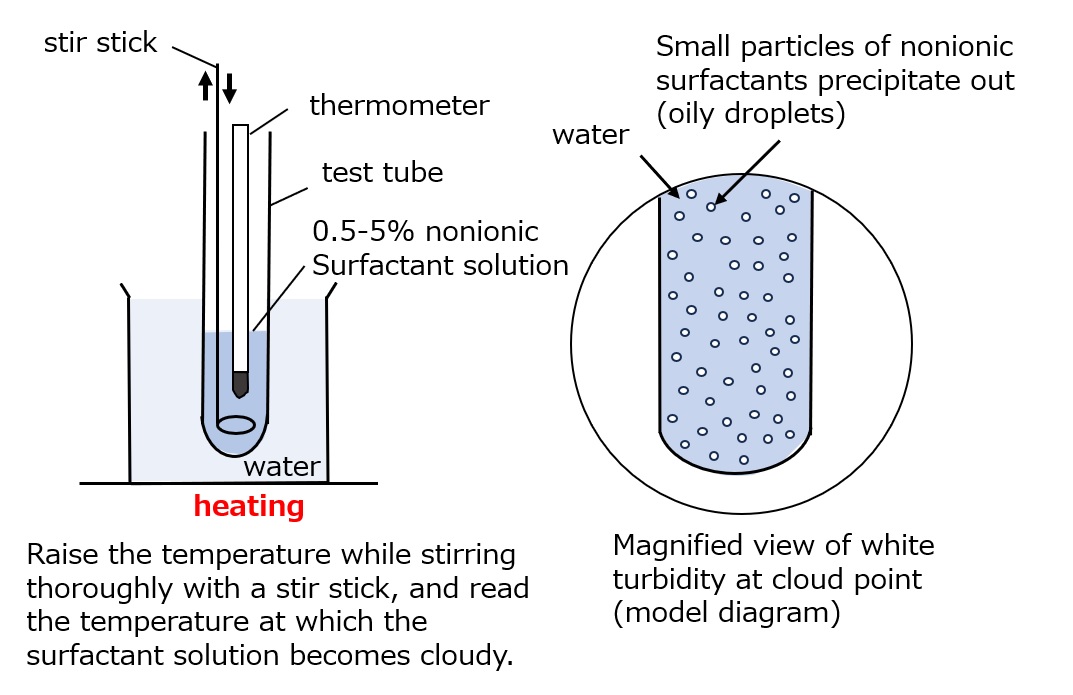
Fig. Method of measuring cloud point
When an aqueous solution of a polyethylene glycol-type nonionic surfactant is heated and the temperature is gradually increased, the bound water molecules are gradually dislodged accordingly, resulting in a gradual decrease in hydrophilicity, and finally the surfactant is no longer soluble in water and precipitates, turning the initially clear solution into a cloudy emulsion.
Thus, when a clear aqueous solution of polyethylene glycol-type nonionic surfactant is gradually heated, the temperature at which the entire solution suddenly becomes cloudy and the surfactant precipitates as fine droplets is called the cloud point.
Relationship between hydrophilicity and cloud point of surfactants
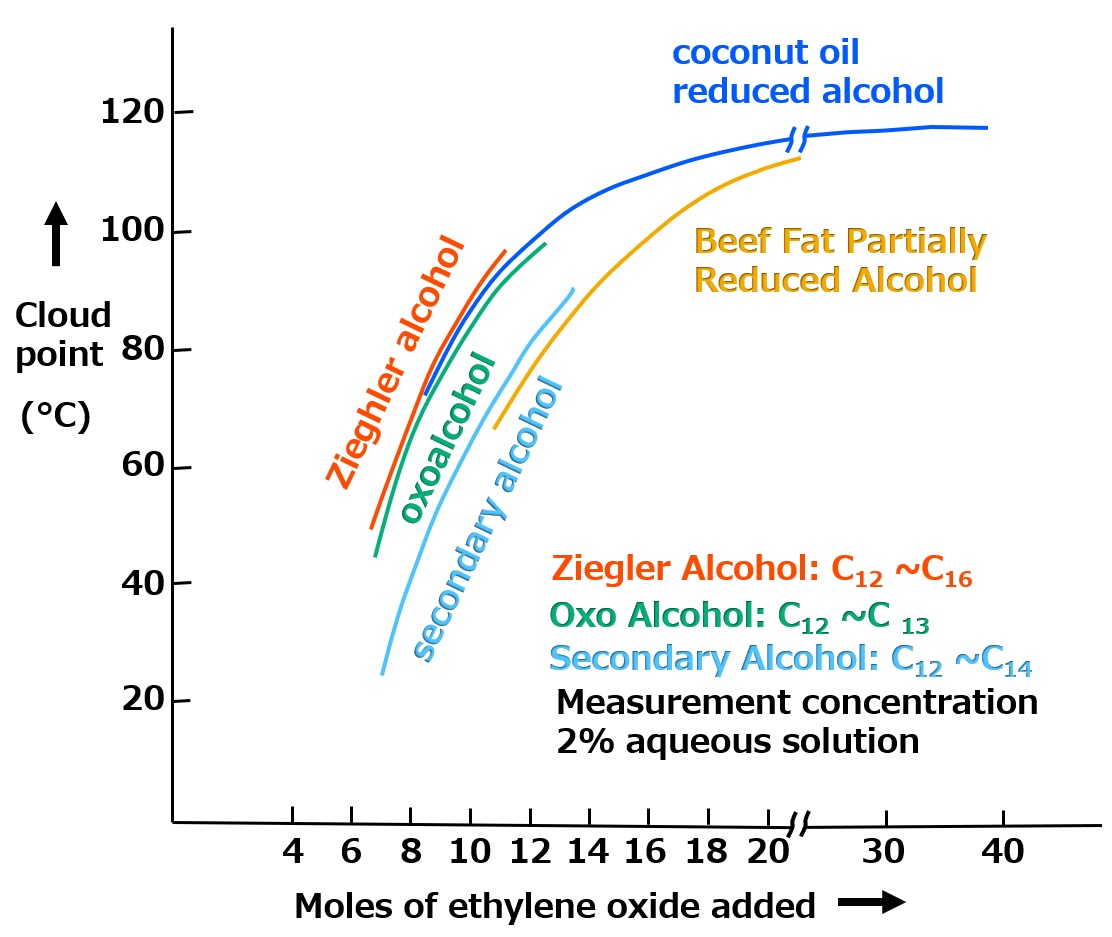
Fig. Relationship between the number of moles of ethylene oxide added and the cloud point (image)
If the hydrophobic group materials are the same, the cloud point will also increase as the hydrophilicity increases with an increase in the number of moles of ethylene oxide added, and this cloud point can be used as a value representing the hydrophilicity of the nonionic surfactant.
The cloud point can be understood as an indication of how strong the hydrophilicity of the polyethylene glycol moiety attached to the hydrophobic group is compared to the strength of the hydrophobic group.
The effect of a surfactant is originally derived from the balance between the opposing properties of the hydrophobic and hydrophilic groups, and the cloud point, which indicates the degree of this balance, is the most important value that determines the properties of polyethylene glycol-type nonionic surfactants.
In fact, quality control of this type of surfactant and guidelines for its use are based on the measurement of the cloud point. For example, it is generally accepted that a surfactant with a cloud point near the operating temperature has excellent permeability. However, the presence of salts or alkalis such as sodium hydroxide causes the cloud point to drop dramatically, so in such cases, it is necessary to measure the cloud point under operating conditions to make a judgment.
Alkylphenol ethylene oxide adducts and higher alcohol ethylene oxide adducts
Nonionic surfactants are obtained by adding ethylene oxide to the phenolic hydroxyl group of alkyl phenols or the alcoholic hydroxyl group of higher alcohols. These are also called polyethylene glycol ether type nonionic surfactants because the hydrophobic and hydrophilic groups are linked by an ether bond (-O-). This type of surfactant is rarely degraded by acids or alkalis.
a. Alkylphenol ethylene oxide adduct (polyoxyethylene alkyl phenyl ether)
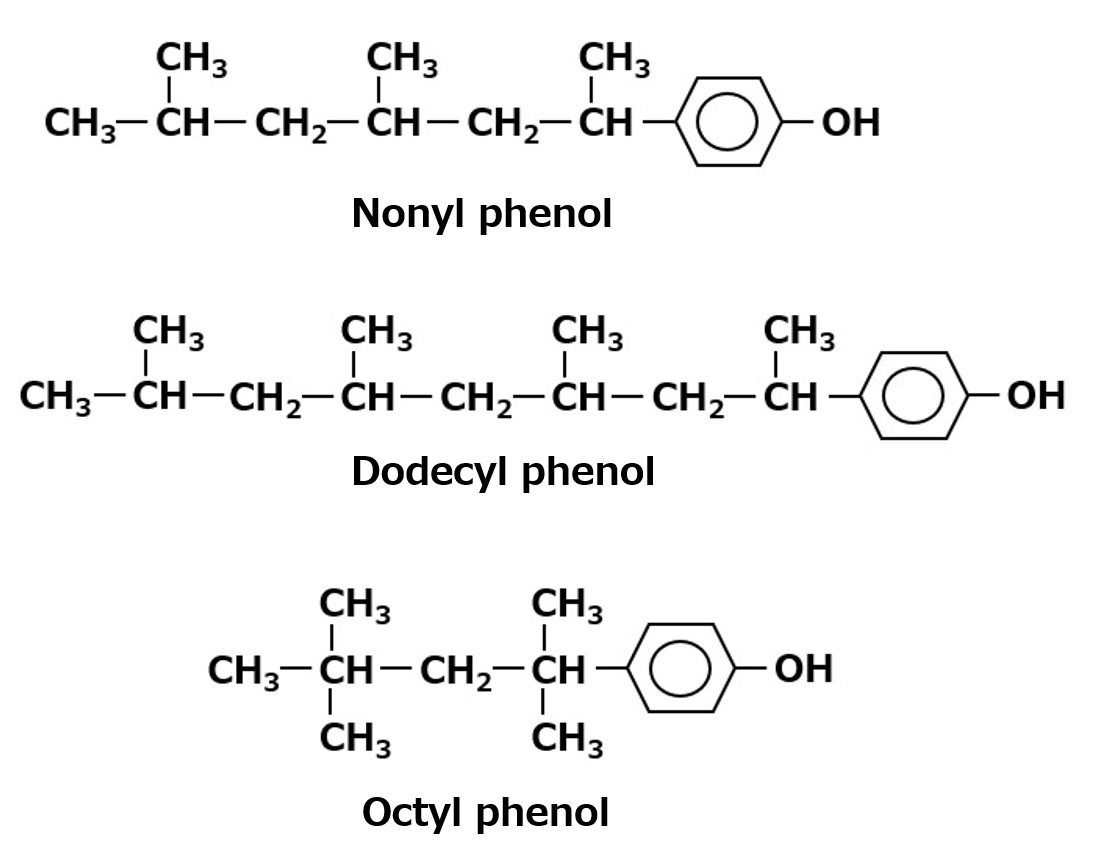
Fig. Typical examples of alkyl phenols
Among alkylphenol EO adducts, nonylphenol, dodecylphenol, octylphenol, octylcresol, and other EO adducts are known.
Among them, nonylphenol EO adducts have been the mainstay of polyethylene glycol ether-type nonionic surfactants because of their superior detergency, penetration, and emulsifying power.
However, alkylphenols have been found to have endocrine disrupting effects, and the use of alkylphenol EO adducts has been declining as they are being replaced by alternative surfactants.
b. Higher alcohol ethylene oxide adduct (polyoxyethylene alkyl ether)
The majority of polyethylene glycol-type nonionic surfactants currently on the market are made by adding ethylene oxide to higher alcohols. Higher alcohols, which are used as raw materials for hydrophobic groups, can be broadly classified into two types: natural alcohols obtained from animal and vegetable oils and waxes, and synthetic alcohols made from petroleum.
Higher alcohols are often used as mixtures of various carbon numbers.
Natural alcohol
Natural alcohols have been replaced by synthetic alcohols for some time due to their generally high price volatility, but their use is now increasing due to environmental concerns and other factors. Generally speaking, C12~C14 alcohols are more suitable as surfactant raw materials than C16~C18.
Typical example of saturated natural alcohol: Coconut oil-reduced alcohol
The most typical saturated natural alcohol is palm oil-reduced alcohol (C12~C14), which is obtained by esterifying palm oil with methanol and reducing the resulting methyl palm oil fatty acid.
Typical examples of unsaturated natural alcohols: palm oil alcohol, olive oil alcohol
Unsaturated alcohols include palm oil alcohol and olive oil alcohol, which are obtained in a similar fashion from palm oil and olive oil, respectively. Both are mixtures of oleyl alcohol (CI8 double bond 1) and cetyl alcohol (C16), among others.
About Natural Alcohols of Animal Origin
Beef fat reducing alcohol (C16~C18) obtained by hydrogenating methyl bovine fatty acid and macko alcohol (C16-C18 double bond 1) obtained by hydrogenating macko whale oil were also used, but are rarely used anymore due to the avoidance of using animal materials and the protection of whale resources. However, it is rarely used anymore due to the avoidance of using animal materials and the protection of whale resources.
Synthetic alcohol
Synthetic alcohols include Ziegler alcohol, which has the same composition as natural alcohols, oxo alcohol, which contains a methyl-branched component, and secondary alcohol, which has a hydroxyl group in the middle of the molecule. All are widely used because they are inexpensive and in stable supply.
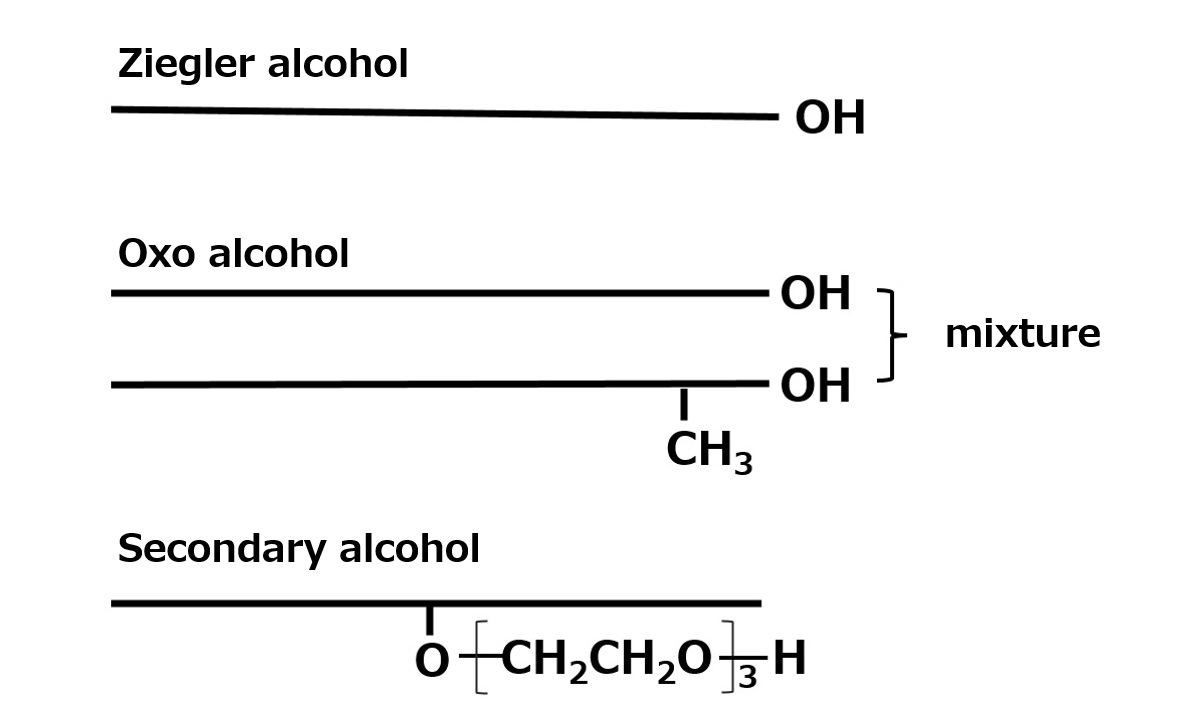
Fig. Comparison of Synthetic Alcohol Structures
Ziegler alcohol
It is made through the process of ethylene polymerization by the Ziegler process. It has a chemical structure (linear primary alcohol) identical to that of saturated natural alcohols.
Oxo alcohol
The reaction of olefin with carbon monoxide and hydrogen yields a primary alcohol with one more carbon atom (oxo method). Although there are some special olefins that use branched-chain olefins such as the trimer and tetramer of propylene as the raw material olefin, the most common method uses linear-chain α-olefins, which are mainly linear primary alcohols like natural alcohols, with some branched primary alcohols mixed in.
Secondary alcohol
It is made by air oxidation of paraffin. It has hydroxyl groups randomly attached to the ends of the carbon chain (linear secondary alcohol). It is usually sold as a 3-mol ethylene oxide adduct. This is because ethylene oxide does not uniformly add under normal reaction conditions without the addition of ethylene oxide.
Higher alcohol ethylene oxide adducts and alkylphenol ethylene oxide adducts
A comparison of polyethylene glycol ether type nonionic surfactants (higher alcohol EO adducts and alkylphenol EO adducts) with anionic surfactants is shown in the table below.
Comparison of polyethylene glycol ether type nonionic surfactants with anionic surfactants
| Properties | anionic surfactant | Polyethylene glycol ether type nonionic surfactant |
|---|---|---|
| Foaming* | Generally good | Generally weak (industrially advantageous) |
| Permeability* | Excellent as dioctyl sulfosuccinated sodium salt | Can be made equal to or better than dioctyl sulfosuccinate sodium salt |
| Detergency* | Generally medium level | Available with high detergency level |
| Emulsification and dispersibility* | Some are quite good. | Freedom to change the number of moles of EO to make it suitable for all areas. |
| Performance as a dyeing aid | Levelling agent for acid dyes, etc. | Levelling agent such as Indanthrene dyes and complex acid dyes |
| Efficacy at low concentrations* | High cmc, so performance drops sharply at low concentrations. | Low cmc, so it performs well even at fairly low concentrations |
| Product form* | Mostly in paste form, some in powder form | Easily made into liquid products (convenient to use) |
Basic Performance of Polyethylene Glycol Ether Type Nonionic Surfactants
| Cloud point [°C] |
Surface Tension [mN/m] |
Penetrating power [sec] |
Detergency [%] |
Foaming degree [mm] |
|||
|---|---|---|---|---|---|---|---|
| Immidiately | After 5min |
||||||
| Synthetic alcohol | Secondary alcohol EO9 mol adduct |
61 | 30 | 2.0 | 28 | 127 | 70 |
| Zeaglar alcohol EO7 mol adduct |
60 | 30 | 3.9 | 32 | 103 | 101 | |
| Oxo Alcohol EO8.5 mol adduct |
63 | 30 | 2.0 | 29 | 115 | 101 | |
| Natural alcohol | Coconut oil reduced alcohol | 61 | 32 | 4.2 | 28 | 114 | 109 |
| Nonylphenol | 61 | 34 | 13.5 | 26 | 72 | 71 | |
| Alkyl phenol | Nonylphenol EO10 mol adduct |
64 | 31 | 2.0 | 30 | 99 | 92 |
Polyoxyethylene fatty acid esters (fatty acid ethylene oxide adducts and polyethylene glycol fatty acid esters)
Ethylene oxide can also be added to fatty acids with an alkali catalyst. This type of product is sometimes called a polyethylene glycol ester-type nonionic surfactant because, as the reaction formula shows, the hydrophobic and hydrophilic groups are linked by an ester bond (-COO-).
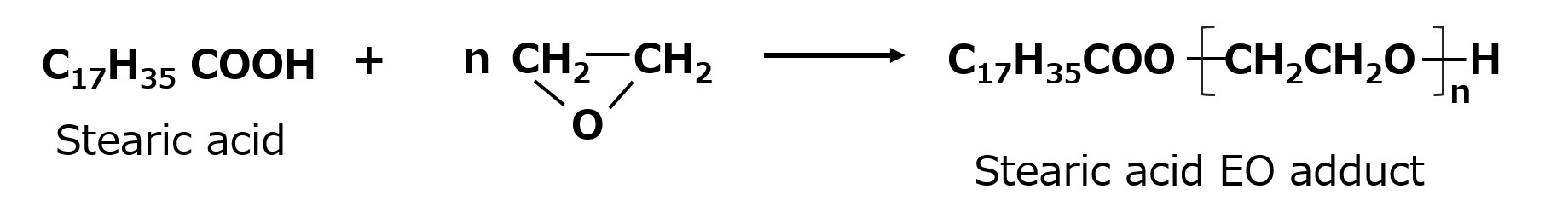
Fig. EO adducts of fatty acids
Since ester bonds are susceptible to hydrolysis, this type of product may decompose into soap when used in strongly alkaline baths. This type of soap is also produced by addition of ethylene oxide as described above, but can also be easily produced by direct esterification of fatty acids with polyethylene glycol.
Polyoxyethylene fatty acid esters are generally inferior to higher alcohols or alkylphenol EO adducts in terms of penetration and detergency. Therefore, they are mainly used as emulsion dispersants, textile oils (for spinning and finishing), or dyeing auxiliaries.
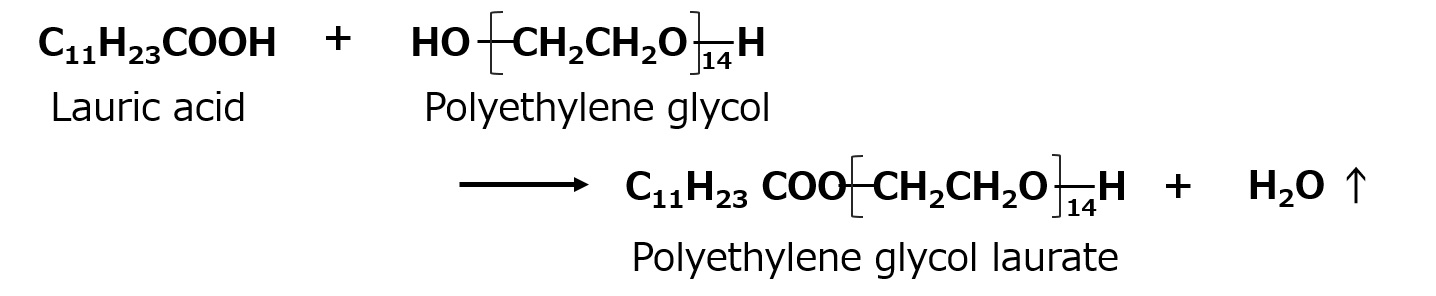
Fig. Polyethylene glycol laurate
Synthesis by Ester Exchange of Fats and Oils with Polyethylene Glycol
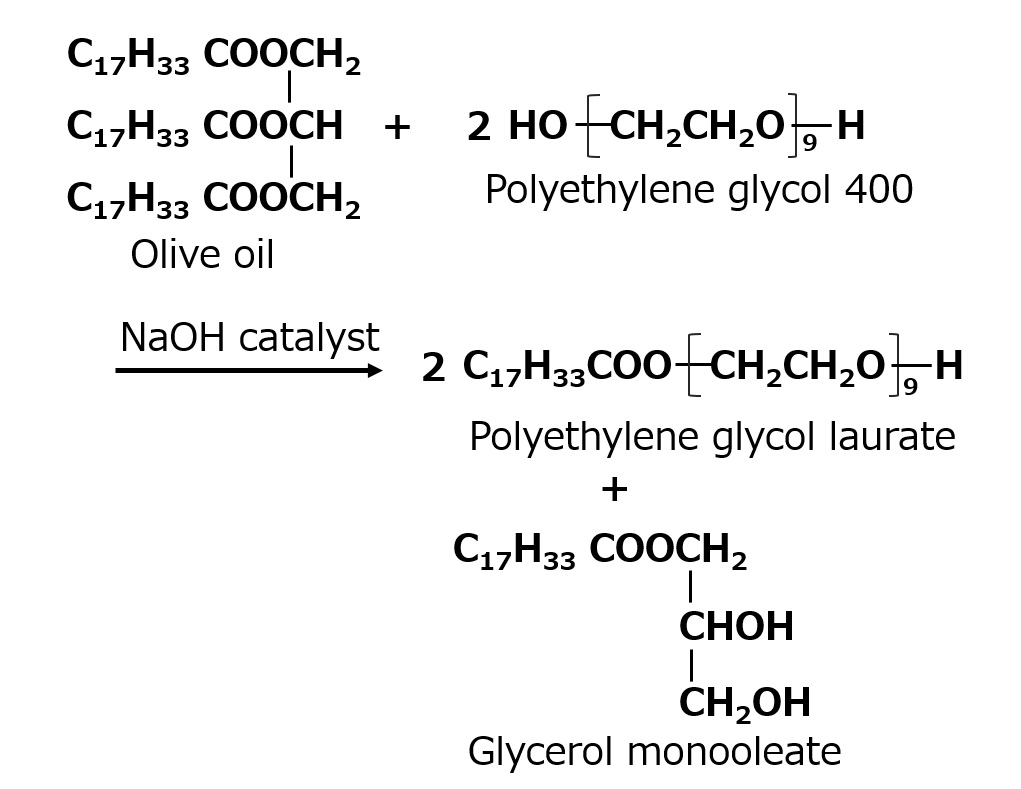
Fig. Glycerol monooleate
To strengthen its characteristics as an oil-soluble emulsifier, polyethylene glycol is added to fats and oils such as olive oil and an alkali-catalyzed ester exchange reaction is performed to make a mixture of polyethylene glycol monooleate and glycerin monooleate, which is also widely used.
However, most are used as raw materials for blending and are not commercially available.
Polyethylene glycol di fatty acid ester
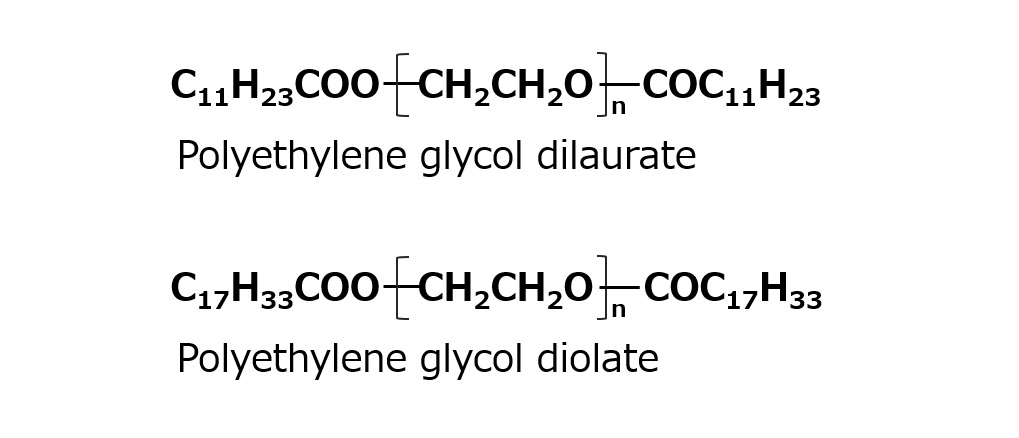
Fig. Diethyl ester type
In addition to polyethylene glycol monofatty acid esters, polyethylene glycol di-fatty acid esters are available as polyethylene glycol ester-type nonionic surfactants.
These diester-type products have extremely low foaming properties and are often used as defoaming agents or low-foaming emulsifiers.
Higher alkyl amine ethylene oxide adducts and fatty acid amide ethylene oxide adducts (polyoxyethylene alkylamine and polyoxyethylene fatty acid amide)
Ethylene oxide can be added also to higher alkylamines or fatty acid amides in the presence of alkaline catalyst.
Higher alkyl amine ethylene oxide adduct
Higher alkyl amines react particularly easily with ethylene oxide, so the reaction can be carried out without a catalyst. In such cases, the polyethylene glycol chain grows after the complete addition of two moles of ethylene oxide to the nitrogen atom first. This type of product has properties intermediate between those of nonionic and cationic surfactants and is used as a dyeing aid.
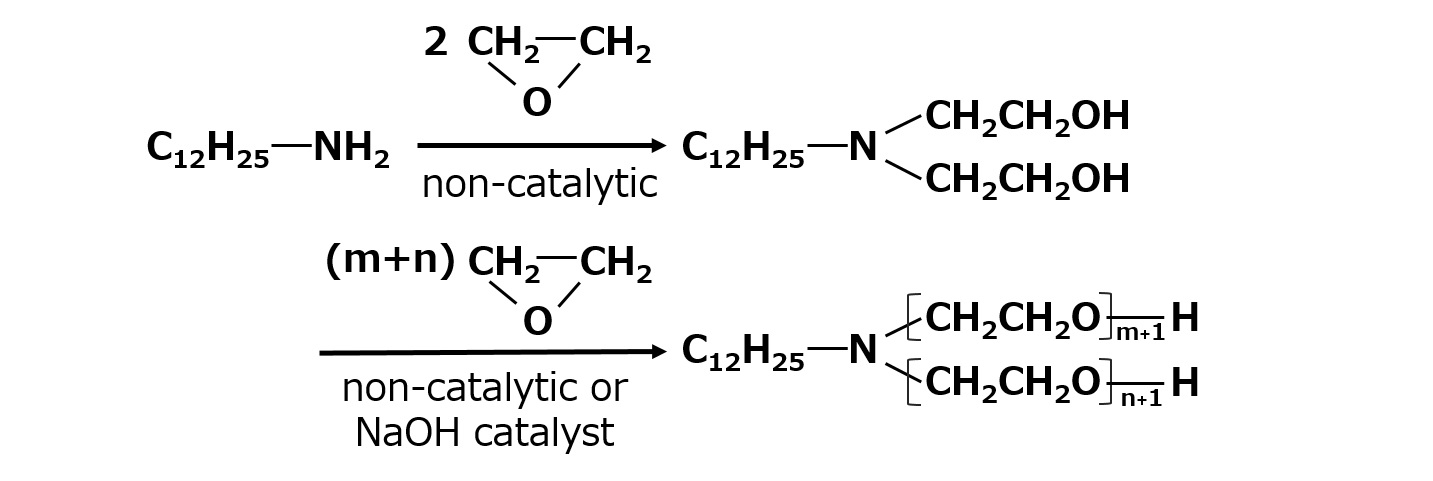
Fig. Higher alkyl amine ethylene oxide adduct
Fatty acid amide ethylene oxide adduct
Fatty acid amides are relatively unreactive with ethylene oxide and usually react as in the following equation, but in reality they are a complex mixture of reactants. In the usual synthesis process, exchange reactions occur during the reaction and the ester and amide bonds are interchanged, resulting in the formation of some of the following compounds, which are nonionic surfactants with somewhat cationic properties. This type of surfactant is used for special applications and is used in relatively small quantities.
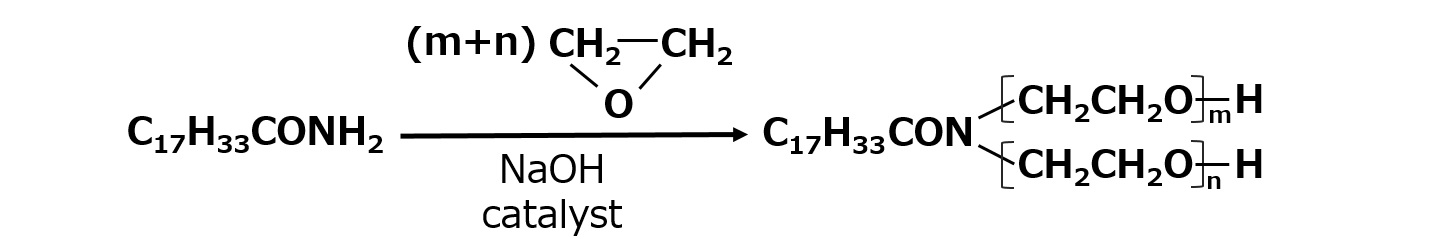
Fig. Fatty acid amide ethylene oxide adduct
Polypropylene glycol ethylene oxide adduct (pluronic nonionic surfactant, polyoxyethylene polyoxypropylene glycol)
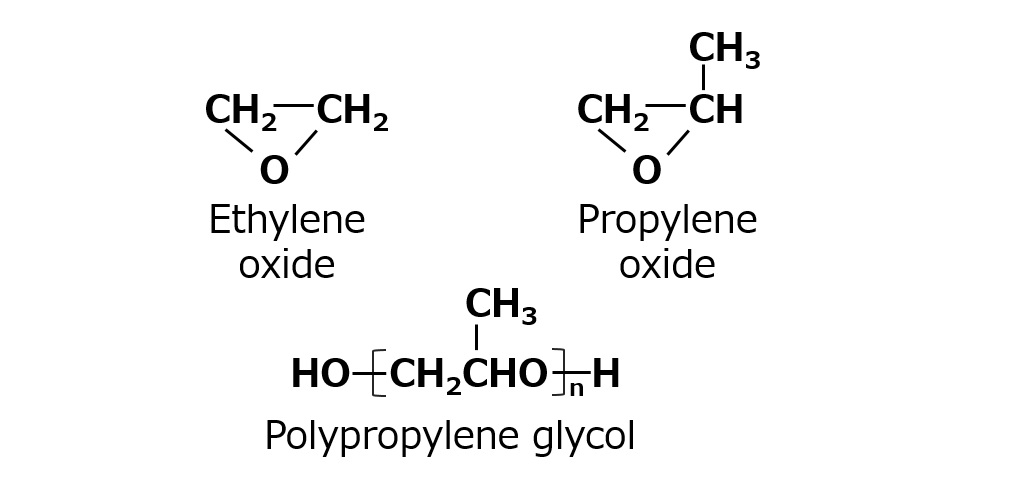
Fig. Structural Formula of Ethylene Oxide, Propylene Oxide and polypropylene glycol structural formula
A compound similar to ethylene oxide is propylene oxide.
Propylene oxide reacts by addition in the same way as ethylene oxide. However, its polymerization product, polypropylene glycol, has a limited water solubility; it is soluble in water up to a molecular weight of several hundred, but insoluble with molecular weight beyond that range. Therefore, polypropylene glycol with a molecular weight of about 1,000 to 2,500 is suitable as a hydrophobic group raw material.
Pluronic type nonionic surfactant
Nonionic surfactants made by adding ethylene oxide to polypropylene glycol were first marketed by the Wyandotte Company in the United States under the trade name "Pluronic" and are therefore called Pluronic-type nonionic surfactants. and are therefore referred to as pluronic nonionic surfactants.
Pluronic-type nonionic surfactants have an unusual shape with hydrophilic groups at both ends with hydrophobic groups in between, as shown in the following formula. Since this type of surfactant has a molecular weight of several thousand, it is much higher in molecular weight than ordinary surfactants (molecular weight of several hundred), so it is sometimes classified as a polymer type surfactant.
Pluronic-type nonionic surfactants are not very promising as penetrating agents due to their molecular weight or molecular shape, but they are used in special applications due to their recognized characteristics as special low-foaming detergents, emulsifying dispersants, viscose additives, and the like.
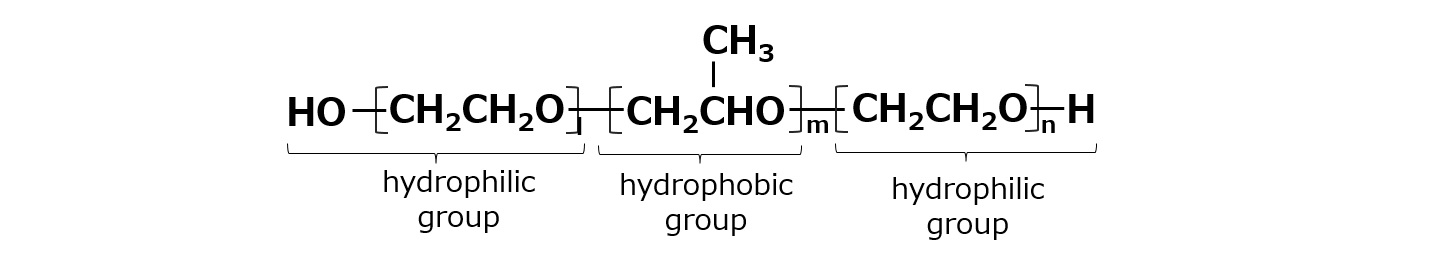
Fig. Structure of Pluronic Nonionic Surfactant
Polyhydric alcohol-type nonionic surfactant
What is a polyhydric alcohol?
Polyhydric alcohols are organic compounds with many alcoholic hydroxyl groups per molecule, such as glycerin, pentaerythritol, and sorbitol.
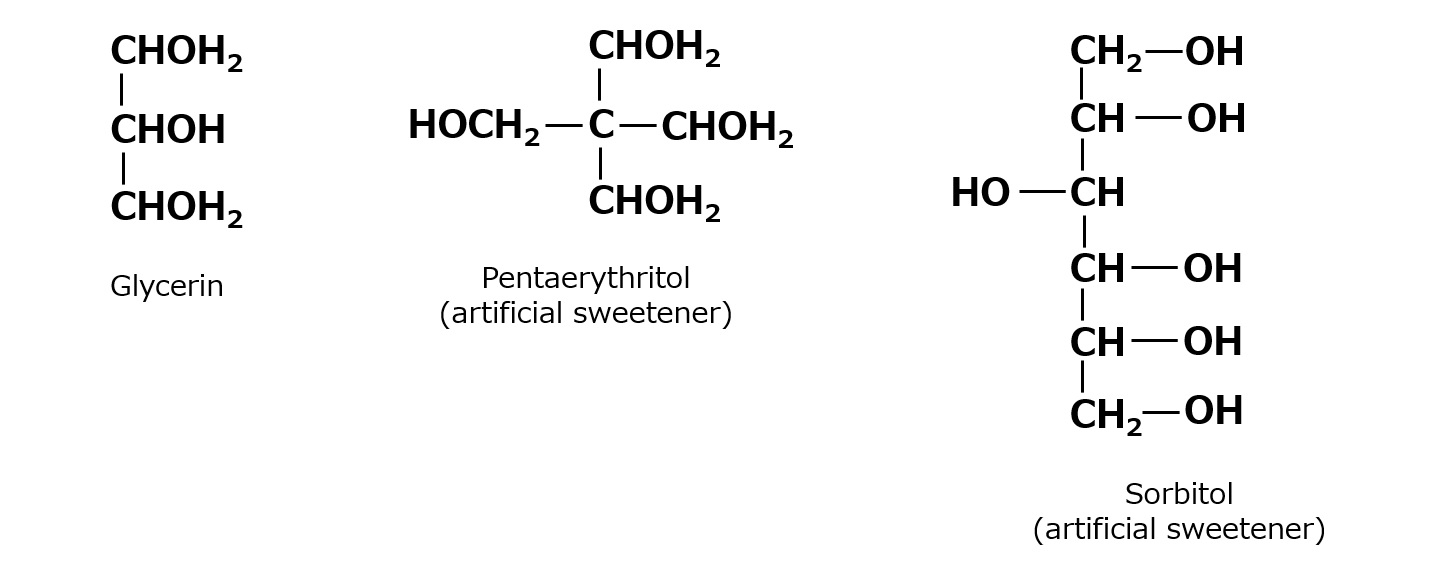
Fig. Example of polyhydric alcohol
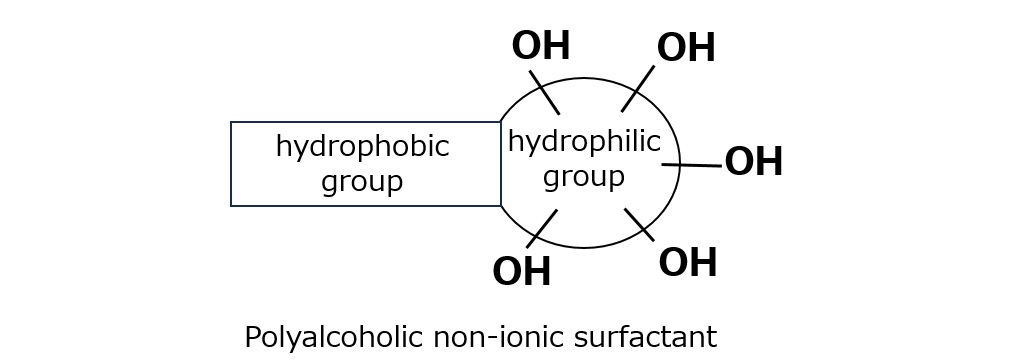
Fig. Polyhydric Alcohol Type Nonionic Surfactants
Polyhydric alcohol-type nonionic surfactants are important surfactants as base agents for textile oils and emulsifiers, and are included in many applied products.
Since polyhydric alcohols have many hydroxyl groups and dissolve well in water, they can be combined with hydrophobic groups such as fatty acids to produce polyhydric alcohol-type nonionic surfactants.
Nonionic surfactants with hydrophobic groups attached to amino alcohols (e.g., diethanolamine) having -NH2 or >NH groups in addition to -OH groups or to saccharides (e.g., glucose) having 1CHO groups are similar to the polyhydric alcohol type. Therefore, they are collectively referred to as polyhydric alcohol-type nonionic surfactants in this section.
The main hydrophilic group materials of polyhydric alcohol-type nonionic surfactants are listed in the table below. Of these, glycerin, pentaerythritol, sorbitan, and diethanolamine are particularly important. Fatty acids are the most commonly used hydrophobic raw materials.
As shown in the table below, many polyhydric alcohol-type nonionic surfactants are not soluble in water, and most are only hydrophilic enough to be emulsified and dispersed in water. Therefore, they are rarely used as detergents or penetrating agents.
Hydrophilic group raw materials for polyhydric alcohol-type nonionic surfactants
| Name | Chemical formula | Solubility of fatty acid esters of amides in water | |
|---|---|---|---|
| polyalcohols | Glycerol OH group number = 3 |
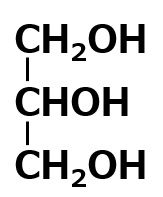 |
Insoluble Self-emulsifying |
| Pentaerythritol Number of OH groups = 4 |
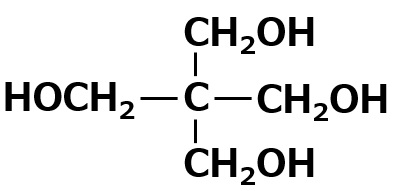 |
Insoluble Self-emulsifying |
|
| Sorbitol Number of OH groups = 6 |
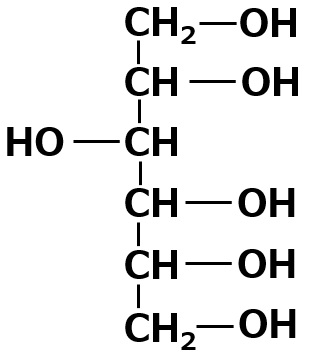 |
Insoluble - scarcely soluble Self-emulsifying |
|
| Sorbitan Number of OH groups = 4 |
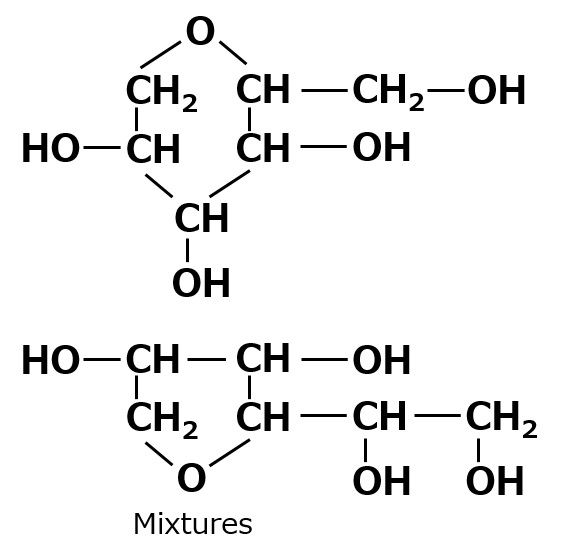 |
Insoluble Self-emulsifying |
|
| Aminoalcohols | monoethanolamine | insoluble | |
| diethanolamine | 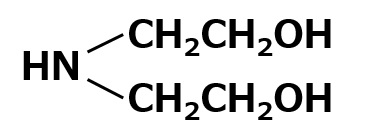 |
1:2 soluble 1:1 is insoluble |
|
| Sugar group | lactose OH group number = 8 |
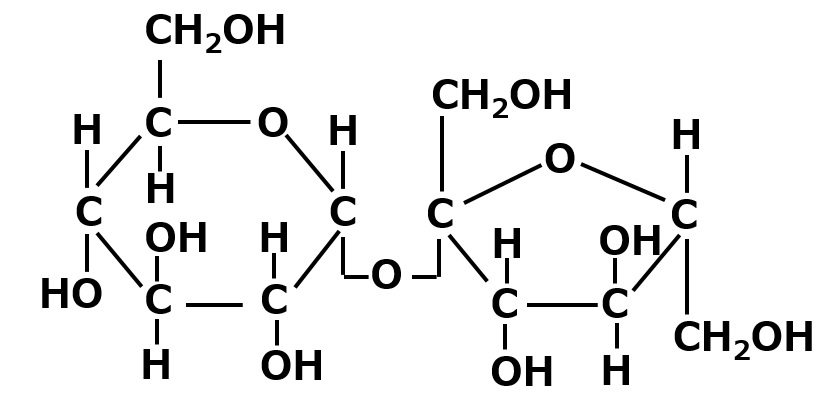 |
Soluble to scarcely soluble |
Fatty acid esters of glycerin and pentaerythritol
The appearance of polyhydric alcohol esters is similar to that of fats, oils, or fatty acids, and they are light yellow solids. Both glycerol esters and pentaerythritol esters are widely used as emulsifiers or raw materials for textile oils (spinning oil or softener), but there are differences in their detailed properties.
Fatty acid esters of glycerin
Glycerol monolaurate or glycerol monostearate is widely used as an emulsifier in food and cosmetics because of its high safety, and especially the technology to produce high-purity products has been developed. They are also used as oils for textiles, but their characteristics as fabric softeners are limited to relatively specialized applications.
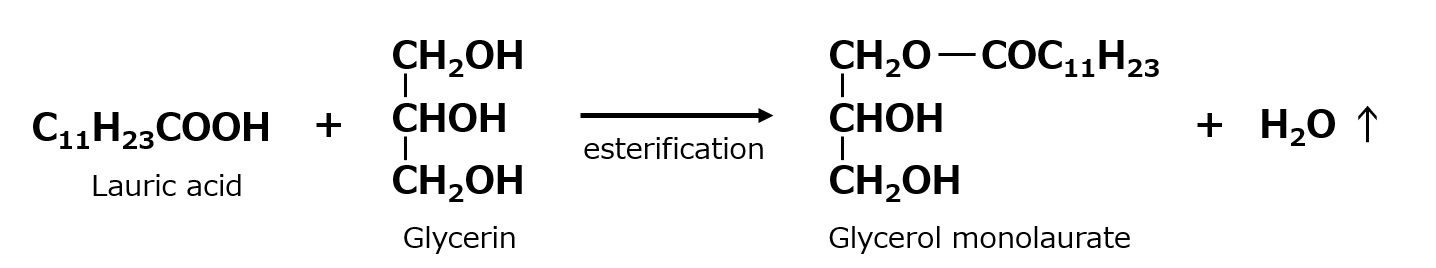
Fig. Glycerol monolaurate
Fatty acid esters of pentaerythritol
Pentaerythritol stearate, for example, is also used as an emulsifier, and is widely used as a base for textile oil formulations due to its excellent softening properties for human silk, staple fibers, and cotton.
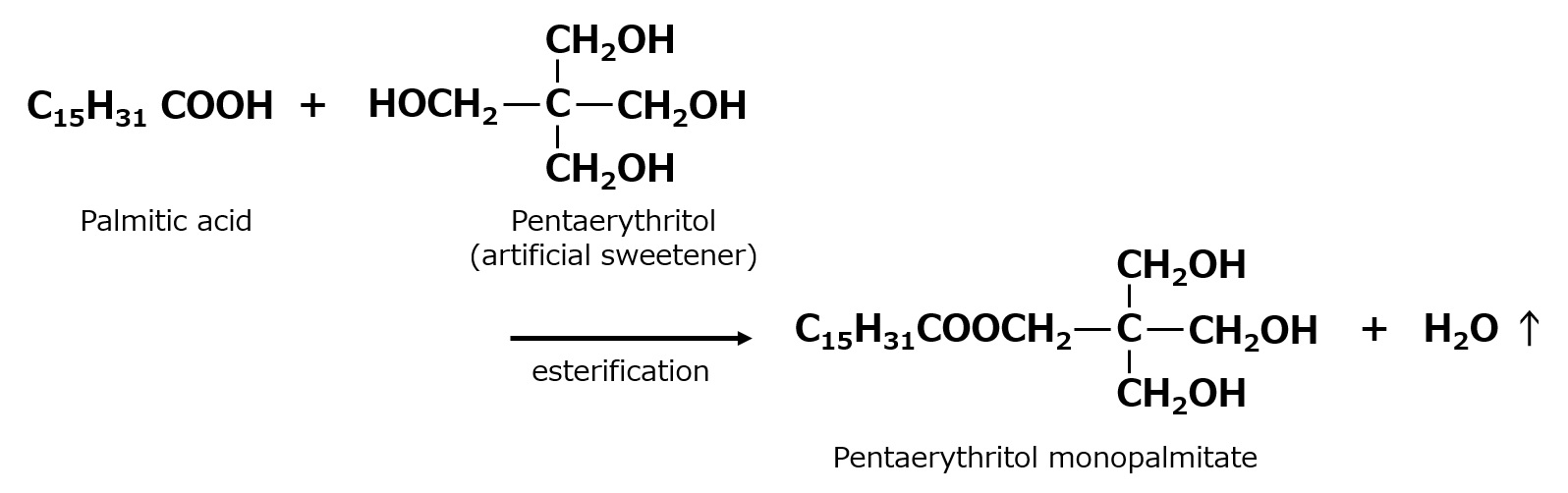
Fig. Example of Pentaerythritol Fatty Acid Esters
Fatty acid esters of sorbitol and sorbitan
Fatty acid esters of sorbitol
Sorbitol is a sweet-tasting polyhydric alcohol produced by reducing glucose with hydrogen and has six hydroxyl groups.
Since sorbitol has no aldehyde groups in its molecule, it is more stable to heat and oxygen than glucose, and there is no risk of decomposition or coloration when reacting with fatty acids.
Sorbitol esters are suitable for textile softeners, but do not work well as general W/O emulsifiers.
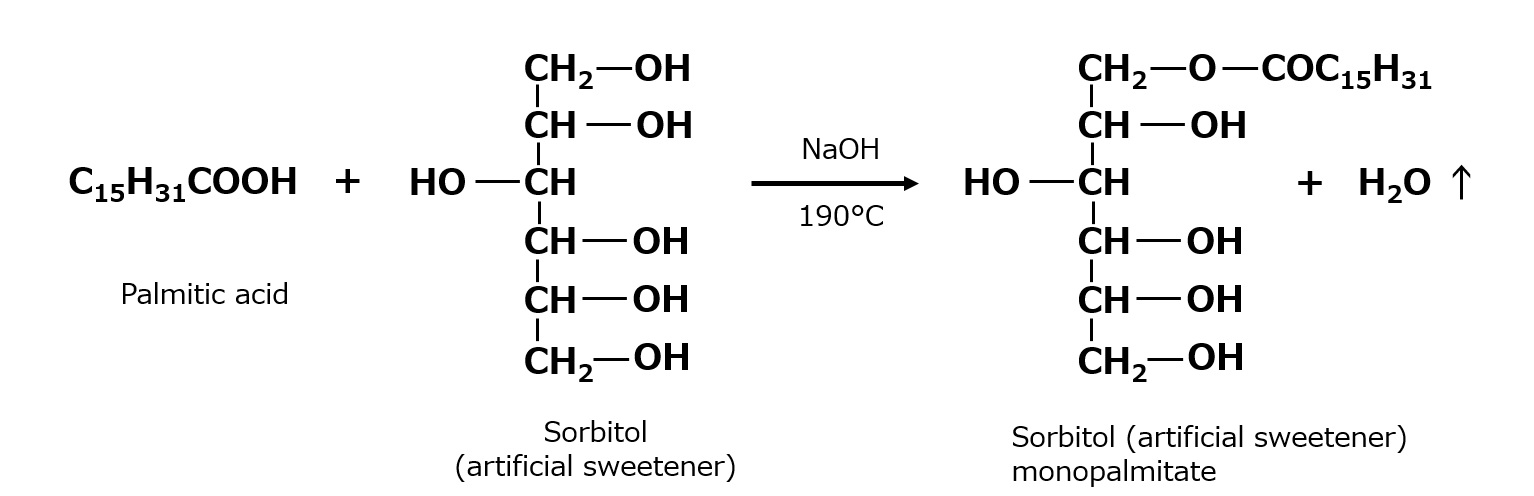
Fig. Example of fatty acid esters of sorbitol
Dehydration-condensation reaction of sorbitol
Sorbitol, when treated under appropriate conditions (e.g., acidic and heated), becomes sorbitan, which is dehydrated one molecule of water, followed by sorbide, through further dehydration of another one molecule of water.
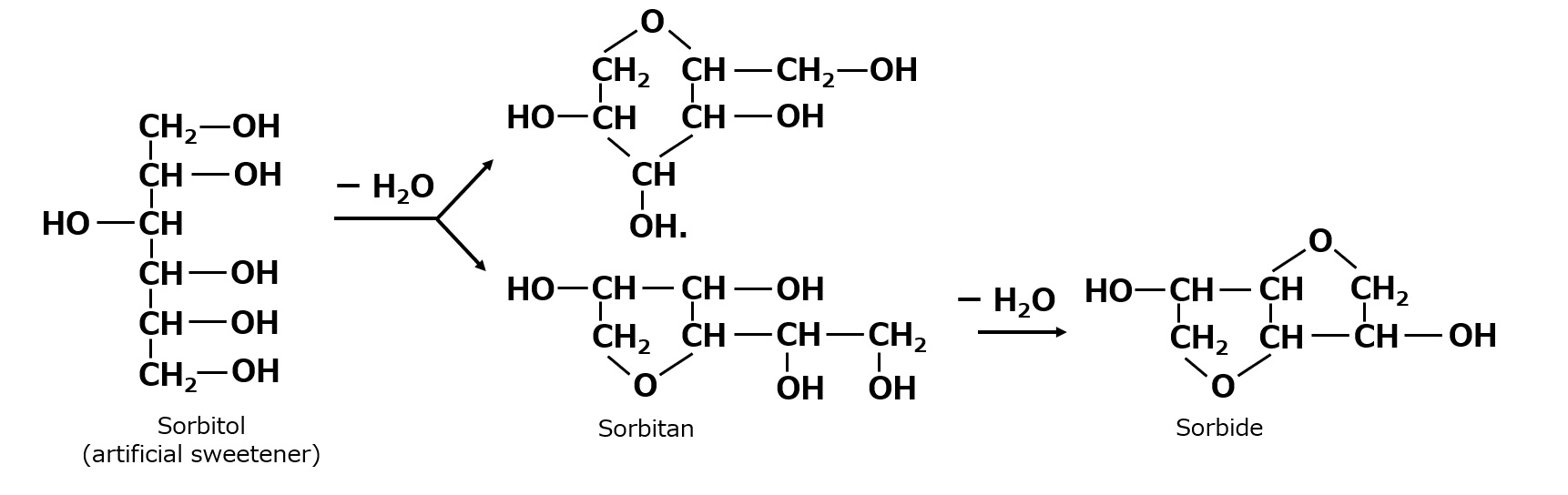
Fig. Dehydration-condensation reaction of sorbitol
Sorbitan is a polyhydric alcohol with four hydroxyl groups, but various isomers are formed depending on the position of the hydroxyl group that reacts when sorbitol is dehydrated. Therefore, what is commonly called sorbitan is a mixture of various sorbitans, not a compound with a single composition. Sorbitan is further dehydrated and has only two hydroxyl groups. In fact, when sorbitol is dehydrated, the reactions shown above occur in a complex manner to produce a mixture of many compounds. Therefore, these sorbitol dehydration products are sometimes collectively called anhydrosorbitols.
Synthesis of sorbitan esters
When the esterification reaction of sorbitol is carried out at 230-250°C, intramolecular dehydration (sorbitanation) of sorbitol also occurs at the same time. If the reaction is stopped after an appropriate time, monopalmitate esters of sorbitan can be obtained in one step.
If the reaction is further continued to proceed with intramolecular dehydration, a product consisting mainly of the sorbitan ester can be obtained.
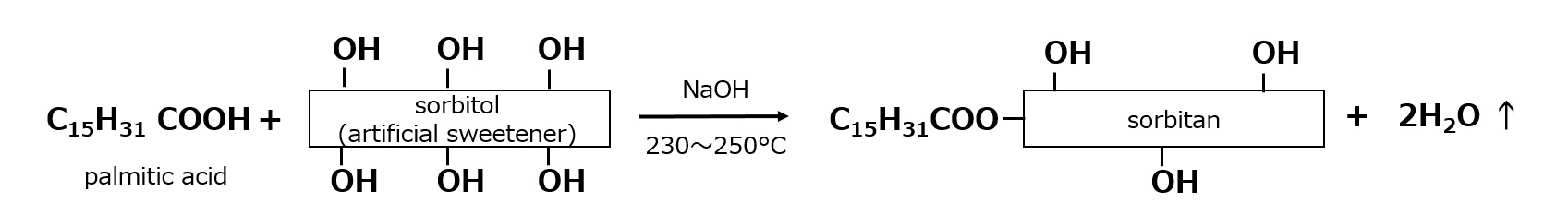
Fig. Example of synthesis of sorbitan ester
Sorbitan Ester Performance and Applications
Sorbitan esters have excellent performance as emulsifiers and textile oils.
Sorbitan ester-type nonionic surfactants are so well known that they are called "spun-type nonionic surfactants" since they were first marketed by Atlas Corporation in the United States under the trade name "Span" (Span) in various varieties.
These sorbitan esters are mainly used as emulsifiers, but since they themselves are almost insoluble in water, they are rarely used alone.
Ethylene oxide adduct of fatty acid esters of sorbitan
A variety of water-soluble emulsifiers are used in sorbitan esters, including a nonionic surfactant with the trade name Tween, which is made by adding ethylene oxide to sorbitan esters.
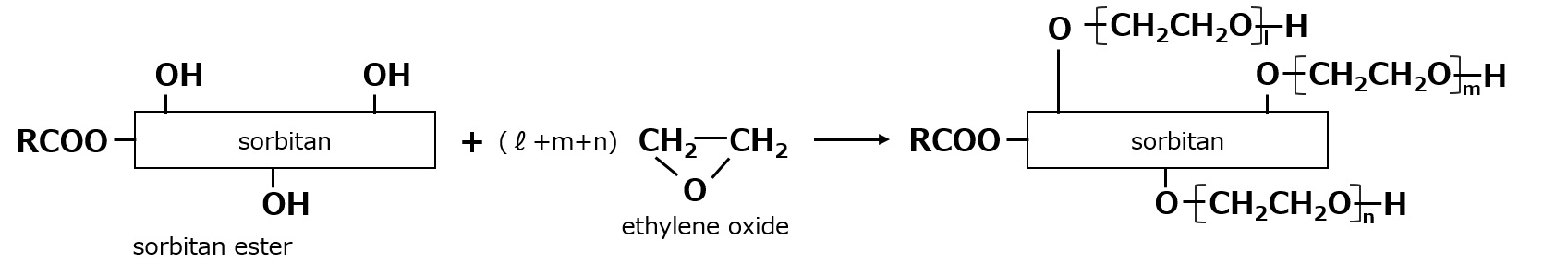
Fig. Ethylene oxide adducts of fatty acid esters of sorbitan
Fatty acid esters of sucrose
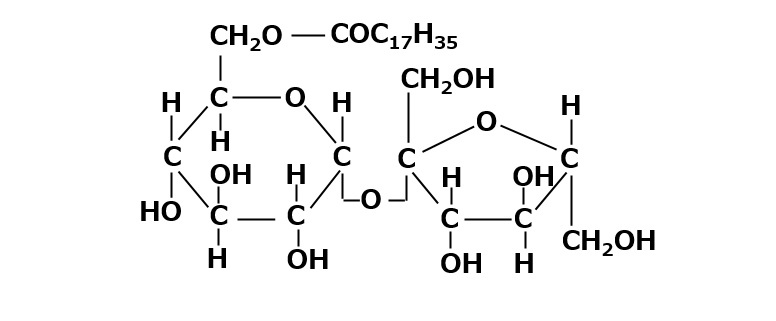
Fig. Monostearic acid esters of sucrose
Among polyhydric alcohol-type nonionic surfactants, fatty acid esters made from hydrophilic materials with three, four, or six hydroxyl groups are almost insoluble in water, but if hydrophilic materials with many hydroxyl groups are used, water soluble products can be made.
As is well known, brown sugar (commonly known as sugar) is a highly hydrophilic raw material with eight hydroxyl groups.
Mono fatty acid esters of sucrose dissolve transparently in water and are useful as low-foaming detergents and emulsifiers. The most important feature of sucrose esters is that they are tasteless, odorless, and safe substances that can be used as food additives and in the pharmaceutical field.
Surface tension of aqueous solutions of various fatty acid monoesters of sucrose
| Fatty acid monoesters of sucrose | Surface tension [mN/m] | |
|---|---|---|
| 0.1% solution | 1.0% solution | |
| monolaurate | 33.7 | 33.4 |
| monomyristate | 34.8 | 33.1 |
| monopalmitate | 33.7 | 33.7 |
| monooleate | 31.5 | 31.8 |
| monostearate | 34.0 | 33.5 |
| sodium dodecyl benzene sulfonate | 29.4 | 31.0 |
Alkyl polyglycoside
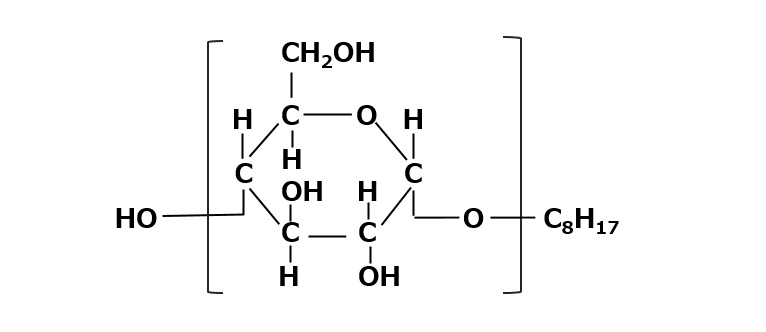
Fig. Alkyl polyglycosides
Alkyl polyglycosides consist of a monosaccharide or an oligosaccharide composed of two or three moles of monosaccharides bonded to an alkyl group.
This type of surfactant is characterized by excellent biodegradability and biocompatibility, as well as high detergency, emulsifying power, and foaming power.
Fatty acid alkanolamide
Fatty acid esters of polyhydric alcohols and sugars are susceptible to hydrolysis. Instead of these esters, those linked by amide bonds are surfactants that are also resistant to hydrolysis. Many polyhydric alcohol-type nonionic surfactants with amide bonds have been synthesized by combining fatty acids with compounds that have amino and hydroxyl groups.
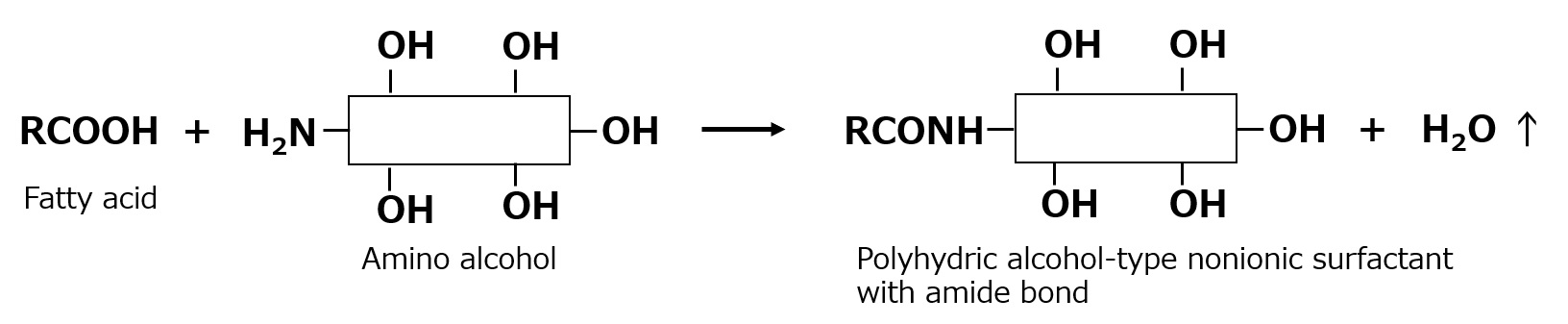
Fig. Fatty acid alkanolamides
Fatty acid esters of polyhydric alcohols and sugars are susceptible to hydrolysis. Instead of these esters, those linked by amide bonds are surfactants that are also resistant to hydrolysis. Many polyhydric alcohol-type nonionic surfactants with amide bonds have been synthesized by combining fatty acids with compounds that have amino and hydroxyl groups.
The most prominent of these polyhydric nonionic surfactants with amide bonds is fatty acid alkanolamide, which is synthesized by the condensation of alkanolamine and fatty acids.
1:2 type fatty acid alkanolamide
Fatty acid alkanolamides were first marketed by the U.S.-based Ninol Corporation and were therefore also called "Ninol-type detergents. This is the product of dehydration-condensation of 1 mole of lauric acid or palm oil fatty acid with 2 moles of diethanolamine.
Although this formula may seem to leave an extra mole of diethanolamine, the extra diethanolamine is actually loosely bound to the produced lauric acid diethanolamide, making the resulting fatty acid alkanolamide very water soluble.
It is also called 1:2 fatty acid diethanolamide because it is produced at a ratio of 2 moles of diethanolamine to 1 mole of fatty acid.
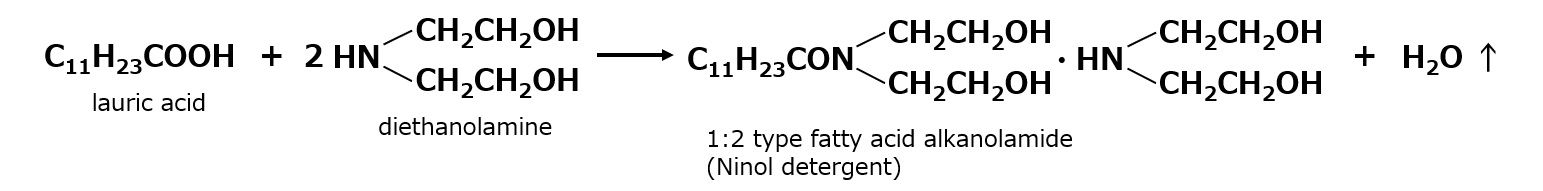
Fig. 1:2 fatty acid alkanolamide (Ninol detergent)
1:1 type fatty acid alkanolamide
The detergency-enhancing and foam-stabilizing effects of 1:2 fatty acid alkanolamides described above are caused by their main component, the fatty acid alkanolamide, and have little to do with the second mole of diethanolamine. Therefore, when added as a foam stabilizer to a highly water-soluble detergent, such as sodium dodecylbenzenesulfonate, the extra diethanolamine is unnecessary, as it is added simply to provide water solubility.
From this perspective, 1:1 type fatty acid diethanolamides without the second mole of diethanolamine were produced for compounding. Lauric acid or coconut oil fatty acid is still used as the fatty acid, but it is usually made into a methyl ester to facilitate the reaction.
This one is widely used as a base for detergent formulations because of its high purity and economic efficiency. A 1:1 type alkanolamide is also made from monoethanolamine and monoisopropanolamine and used for similar purposes.
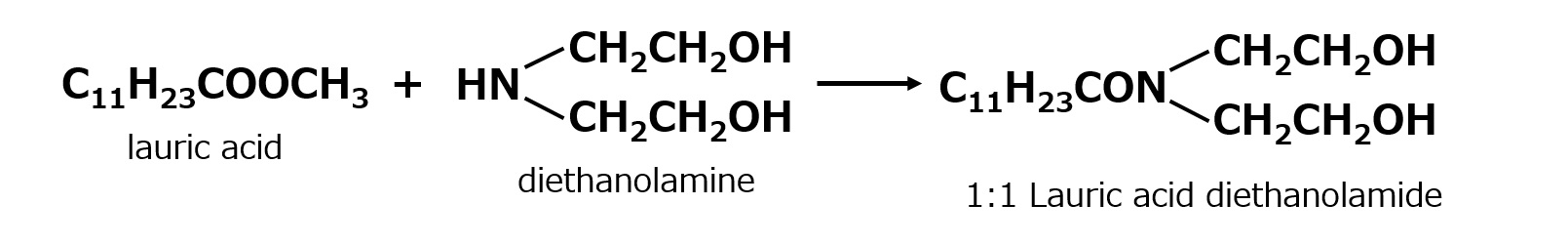
Fig. 1:1 Fatty Acid Diethanolamide
Summary of Nonionic Surfactants
Two very different types of nonionic surfactants with very different properties
Many polyethylene glycol-type nonionic surfactants are well soluble in water and are mainly used as detergents, dyeing aids, and emulsifiers,
Many polyhydric alcohol-type nonionic surfactants are insoluble in water and are mainly used as fabric softeners and emulsifiers. Nonionic surfactants are classified by hydrophobic and hydrophilic group materials as shown in the table below.
Classification of polyethylene glycol-type nonionic surfactants by raw material
| Hydrophilic group raw material | |||
|---|---|---|---|
| ethylene oxide | polyethylene glycol | ||
| Hydrophobic group raw material |
higher alcohol | Polyethylene glycol ether (detergent, emulsifier) |
- |
| alkyl phenol | Polyethylene glycol ether (detergent, emulsifier) |
- | |
| fatty acid | Polyethylene glycol ester (emulsifier, oil) |
Same as on the left. | |
| higher alkyl amine | (Dyeing aids, etc.) | - | |
| fatty acid amide | (Special use) | - | |
| fats and oils | (Emulsifiers, special applications) | (Emulsifier) | |
| fatty acid esters of sorbitan | Tweens (Emulsifier) |
- | |
Classification of Polyhydric Alcoholic Nonionic Surfactants by Raw Material
| Hydrophilic raw materials | ||||||
|---|---|---|---|---|---|---|
| glycerin | pentaerythritol (artificial sweetener) |
sorbitol, sorbitan, etc. |
dehiscent fruit | alkanolamine | ||
| Hydrophobic raw material | fatty acid | Glycerol Mono fatty acid esters |
Polyhydric alcohols Ester (Oils) |
Polyhydric alcohols Ester (emulsifiers, oils) |
lactose esters (detergent, emulsifier) |
Alkanolamide (Detergent, foam stabilizer) |
| fats and oils | Glycerol Mono fatty acid esters (emulsifier, oil) |
Mixed polyhydric alcohols Ester (Oils) |
- | - | (Special use) | |
Of the raw materials shown in this table, ethylene oxide is produced inexpensively due to the development of petrochemistry. In addition to those derived from natural products, a wide variety of synthetic higher alcohols have also appeared on the market.
Furthermore, considering the excellent performance and versatility of polyethylene glycol-type nonionic surfactants, this type of product is likely to become increasingly important in the future.
In addition to those listed in the table above, there are also higher alkyl mercaptans (R-SH) as hydrophobic group materials and dipentaerythritol and polyglycene as hydrophilic group materials, but these are omitted in this section.
Reference: "Introduction to Surfactants" by Takehiko Fujimoto, Sanyo Kasei Kogyo (2014)
Related products(surfactants)
Surfactants for laundry detergents and kitchen detergents

- Nonionic surfactant
Nonionic Surfactants Catalog
A list of properties of Sanyo Chemical's representative nonionic surfactants.

- Nonionic surfactant
Surfactant, antimicrobial agent, dispersant: categoly page
A list of properties of Sanyo Chemical's representative nonionic surfactants.

- Nonionic surfactant
Low-foaming nonionic surfactant for machine and metal cleaning "SEDORAN FF"
Low foaming and good foam breakability make it suitable for machine and metal cleaning by spraying or jet washing.

- Nonionic surfactant
Polyoxyethylene - Polyoxypropylene Block Copolymer ”NEWPOL PE”
Lineup of products with distinctive features and a wide variety of functions can be added

- Nonionic surfactant
Polyoxyalkylene alkylamine-based surfactant "PUREMEEL EP-300S"
Base detergent for clothes with excellent cleaning power against grease and oil stains
Related topics


This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.





