Introduction to Anionic Surfactant
What is a surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
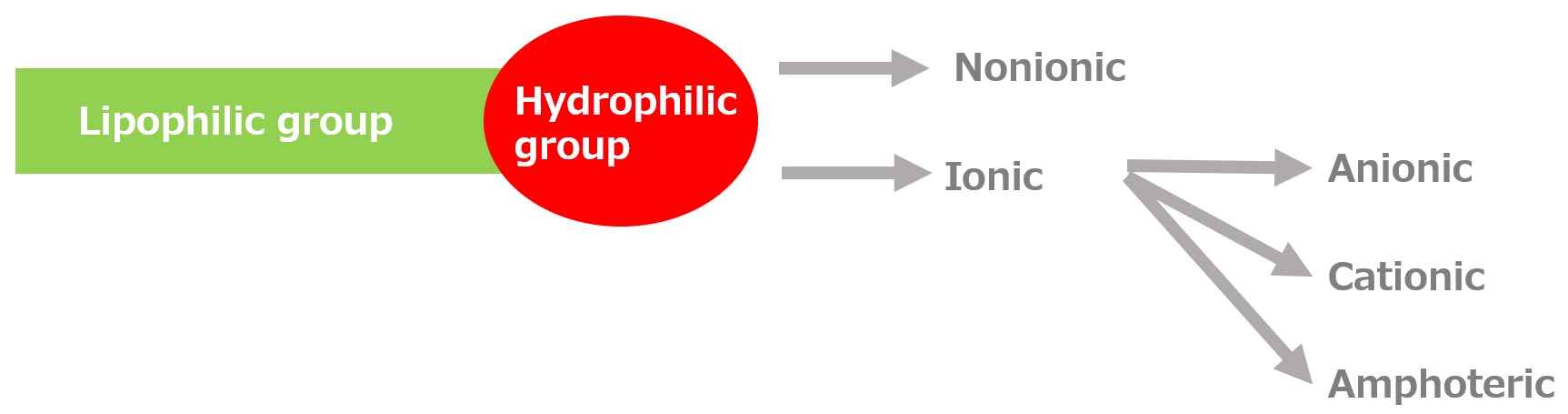
Basic structure of a surfactant
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-loving parts) and hydrophilic groups (water-loving parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant |
-Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH |
-Laundry detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil |
-Polyoxyethylene alkyl ether etc. |
| Anionic surfactant |
-Excellent emulsification and dispersibility -Good lather -Temperature insensitive |
-Laundry detergent -Shampoo -Body soap |
-Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant |
-Adsorption to fibers -Antistatic effect -Bactericidal |
-Hair conditioner and treatment -Fabric softener -Disinfectant |
-Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant |
-Mild on skin -Excellent solubility in water -Synergistic with other active components |
-Shampoo -Body soap -Kitchen detergent |
-Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant functions introduction video
It consists of seven short movies for each function.
0:00 Introduction of surfactants functions
0:16 Part1 Washability
1:00 Part2 Permeability
2:10 Part3 Dispersion
2:55 Part4 Foaming properties
3:25 Part5 Defoaming properties
3:39 Part6 Smoothness
4:20 Part7 Antibacterial properties
What is anionic surfactant?
Anionic surfactants have been used for a long time and are still the second most commonly used surfactants after nonionic surfactants.
The history of surfactants begins with the widespread use of soap, which replaced traditional methods of washing clothes with lye and similar substances. Turkey red oil (sulfated castor oil) followed as a pioneer of synthetic surfactants, and its efficacy was praised for a long time in the dyeing and finishing of textiles industry. Over time, the surfactant industry evolved, with the introduction of salts higher alcohol sulfates, alkanoyl methyl tauride, and alkyl benzene sulfonates, laying the foundation for the modern range of anionic surfactants.
First, anionic surfactants are classified as shown in the table below.
Classification of anionic surfactants
| Carboxylate | Soap |
| Polyoxyethylene alkyl ether carboxylate | |
| Alkyl hydroxy ether carboxylate | |
| Other | |
| Sulfonate | Alkyl benzene sulfonate |
| Alkylnaphthalene sulfonate | |
| Paraffin sulfonate | |
| Alkanoylmethyltauride | |
| Dialkyl sulfoammonate | |
| Other | |
| Carboxylates/sulfonates | Alkyl sulfoammonate disodium salt |
| Polyoxyethylene alkyl ether sulfoammonate disodium salt | |
| Sulfate | Higher alkyl sulfates (higher alcohol sulfates) |
| Polyoxyethylene alkyl ether sulfates (Higher alcohol EO adduct sulfates, higher alkyl ether sulfates) |
|
| Sulphated oil | |
| Sulfated fatty acid esters | |
| Sulfated olefin | |
| Other | |
| Phosphate ester | Higher alkyl phosphates (higher alcohol phosphates) |
| Polyoxyethylene alkyl ether phosphate salts (Higher alcohol EO adduct phosphates, higher alkyl ether phosphates) |
|
| Dithiophosphate | |
| Other |
Carboxylate
Soap
When natural oils and fats are stirred together with an aqueous solution of sodium hydroxide (generally an alkaline solution) while heating, they react (saponification) to form soap (i.e., alkali metal salts of higher fatty acids) and glycerin.
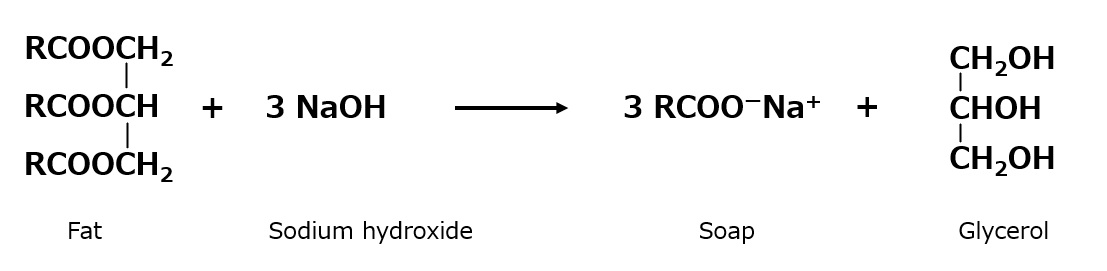
Fig. Saponification reaction of fats and oils
When a concentrated solution of salt is added, the soap precipitates (salting out) and floats to the top, where it is extracted, refined, and marketed. Since sodium hydroxide is the most commonly used alkali, the word "soap" usually refers to sodium soap, but potassium hydroxide is sometimes used specially for cosmetics, etc. In this case, potassium soap is used in a soft state containing glycerin without salting out. Potassium hydroxide is also used for cosmetic purposes.
The raw material oils and fats used include beef tallow, coconut oil, palm oil, rice bran oil, soybean oil, peanut oil, and hydrogenated oil, with coconut oil and palm oil being the most common in terms of volume. Different types of raw material oils and fats contain different types of fatty acids and their ratios, so the performance of the resulting soaps will vary. The following is a comparison of the properties of typical fatty acid soaps.
Sodium laurate: C11H23COO-Na+
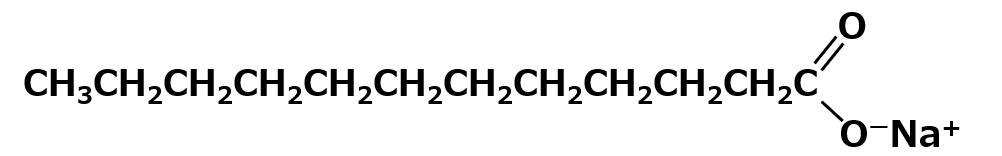
It is the main ingredient in soap made from coconut oil, and has a relatively short hydrophobic group with a total of 12 carbons. This makes it easily soluble in water and has excellent detergency (when the carbon number is less than 12, water solubility becomes better, but detergency becomes worse).
Sodium stearate: C17H35COO-Na+
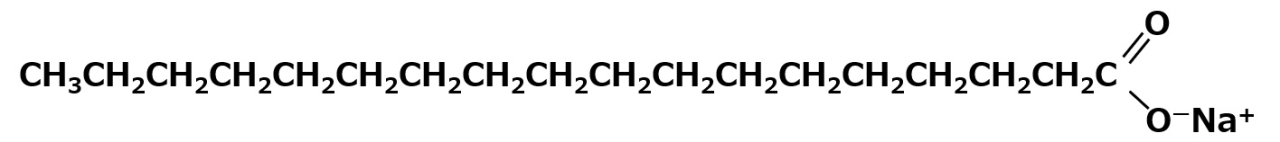
It is an 18-carbon saturated fatty acid soap that is commonly found in soaps from solid fats and oils such as hydrogenated oil. The long hydrophobic groups and significant hydrophobicity make it difficult to dissolve in water. Even with the hydrophilic carboxyl group (COO- Na+), the sodium salt is not sufficiently hydrophilic for good water solubility.
Sodium Oleate: C17H33COO-Na+
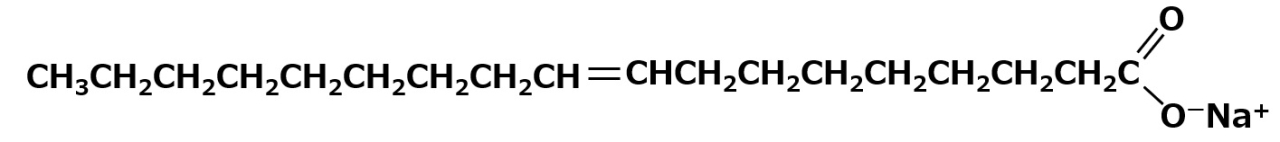
Sodium oleate is the main ingredient in soap made from olive oil, etc. Most beef tallow soap is also made from sodium oleate.
Like stearic acid, it has a total of 18 carbons, but its properties are different because of the double bond in the middle of the molecule. Although, the double bond is weak, it has an affinity with hydrophilic groups. For this reason, sodium oleate dissolves well in water and also has excellent detergent properties.
(Having too strong a hydrophilic group in the middle of the molecule reduces detergency. Castor oil soap, for example, has a hydroxyl group in the middle of the molecule in addition to the double bond, making it soluble in water but not suitable for cleaning.)
Soap solubility/acid effects
Soap is generally less water soluble, so even a slightly higher concentrated solution, it will become viscous and forms gel when left standing. Soap solution is alkaline in nature and loses detergency under neutral conditions, and fatty acids are formed under neutral or acidic environments.

Fig. Reaction of soap and acid
Effect of hard water on soap
In addition, when hard water (water containing high levels of calcium and other salts) is used, water will produce insoluble calcium soap and other substances that will precipitate out, rendering it ineffective.
Therefore, it is difficult to use soap with hard water (those who have experience using soap in hot springs will immediately understand this). In Europe and the U.S., in particular, there are many areas with hard water, making it difficult to use soap, which is why synthetic detergents, which are stable in hard water, were developed early on.

Fig. Reaction of soap and Ca ion
Polyoxyethylene alkyl ether carboxylate
Polyoxyethylene alkyl ether carboxylate is an anionic surfactant that has a nonionic hydrophilic polyethylene glycol chain in addition to the carboxylate, which is a hydrophilic group in soap.
While polyoxyethylene alkyl ether carboxylate is biodegradable and environmentally friendly, it is also more hydrophilic than soap due to its nonionic polyethylene glycol chain. This makes it less irritating to the skin, which is a disadvantage of soap, and it does not produce soap scum, which causes stains on wash basins and other surfaces. Taking advantage of these features, products with a low number of moles of ethylene oxide added (n≤3 in the formula below) are often used in body shampoos, facial cleansers, and hypoallergenic shampoos. They can also be used as detergent base under acidic conditions where soap cannot be used.
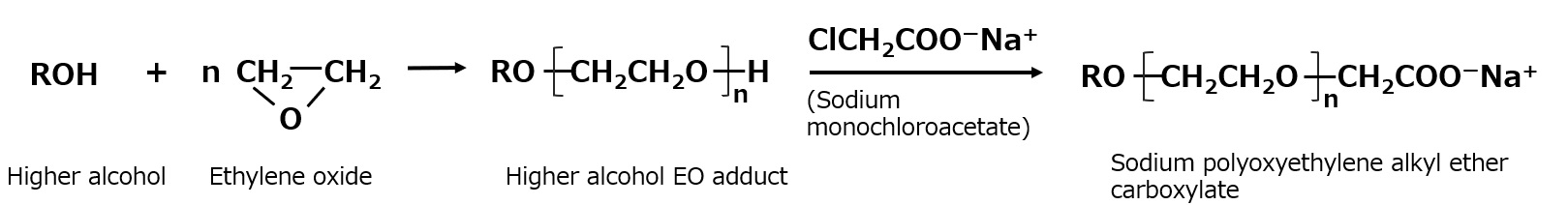
Fig. Synthesis route of sodium polyoxyethylene alkyl ether carboxylate
Alkyl hydroxy ether carboxylate
It is a new anionic surfactant that has a hydroxyl group in addition to the carboxylate, the hydrophilic group of soap.
It is mildly acidic to neutral, which is within the pH range of the skin, and produces fine, high-quality foam. It also has moderate detergency, so it does not excessively remove oil from the skin or hair, is hypoallergenic, and is highly biodegradable, making it suitable as a base for body shampoo and hair shampoo.
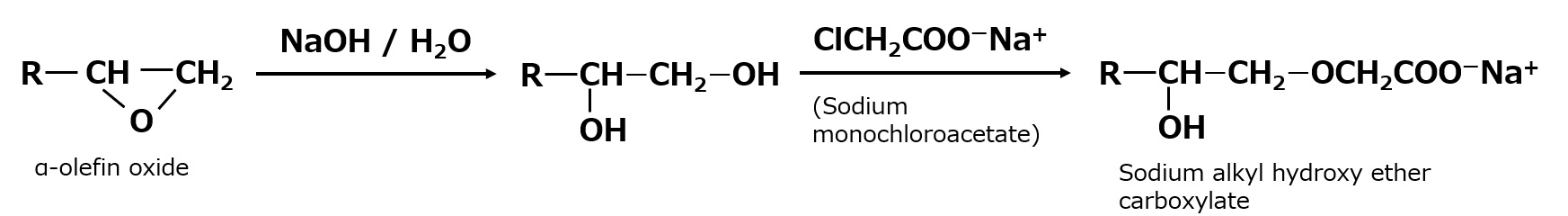
Fig. Synthetic route to sodium alkyl hydroxy ether carboxylate
Sulfonate
Sulfonates are generally represented by R-SO3- Na+ . A similar compound is the sulfate ester salt R-O-SO3- Na+ . Both are obtained by reacting with sulfuric acid, but the reaction to obtain sulfonic acid is sulfonation, while the reaction to obtain sulfate esters is sulfation. Both reactions are important for surfactants in that they introduce hydrophilic groups.
Sulfonates and sulfates are very similar compounds, but they differ in some of their properties, which we know is due to the -O- bond between R (i.e., carbon atom) and S (sulfur atom).
The most important difference is that sulfonates are not hydrolyzed, whereas sulfates are hydrolyzed to their original alcohol and sulfuric acid form when acidified.
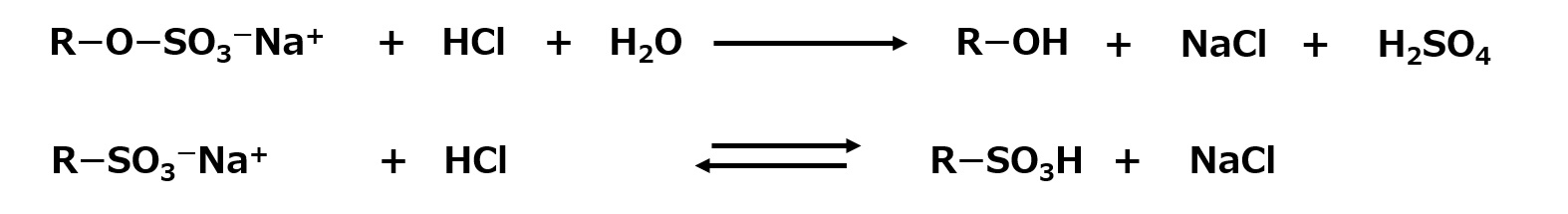
Fig. Reaction of sulfates and sulfonates with acids
Therefore, sulfonates can be used in acidic solutions without problems. In addition, sulfonates are often advantageous because they are less susceptible to decomposition than sulfates when heated.
Sulfonate surfactants include alkylbenzenesulfonates, alkanoylmethyltaurides, and dialkylsulfoammonates.
Alkyl benzene sulfonate
Sulfonation reaction
Sulfonation of aromatic hydrocarbons such as benzene by sulfuric acid or fuming sulfuric acid immediately comes to mind.
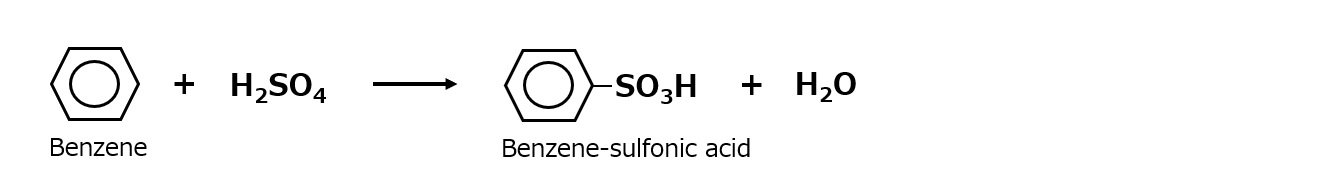
Fig. Sulfonation reaction of benzene
Using this reaction, a surfactant can be made by attaching a long alkyl group, such as a dodecyl group (CI2H25-), to benzene and sulfonating it.
In fact, this reaction proceeds smoothly to produce a variety of surfactants, which are collectively called alkylbenzene sulfonates.
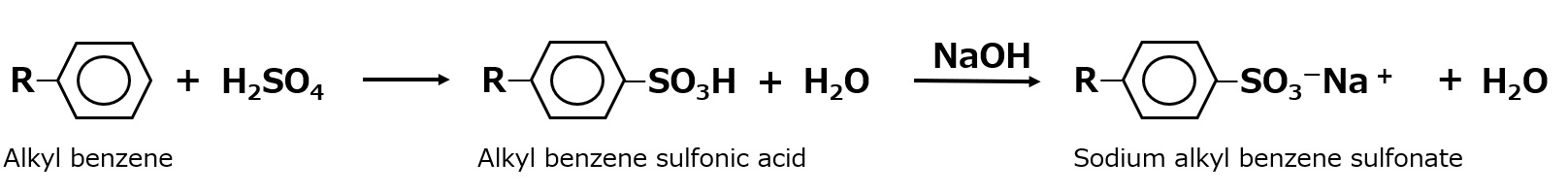
Fig. Synthetic route of sodium alkylbenzenesulfonate
In industrial sulfonation, fuming sulfuric acid or anhydrous sulfuric acid, which has a much higher concentration than concentrated sulfuric acid, is used as a sulfonating agent to make the reaction smooth and eliminate the use of extra sulfuric acid. Mass production is carried out in large plants with fully automated controls that use liquid anhydrous sulfuric acid.
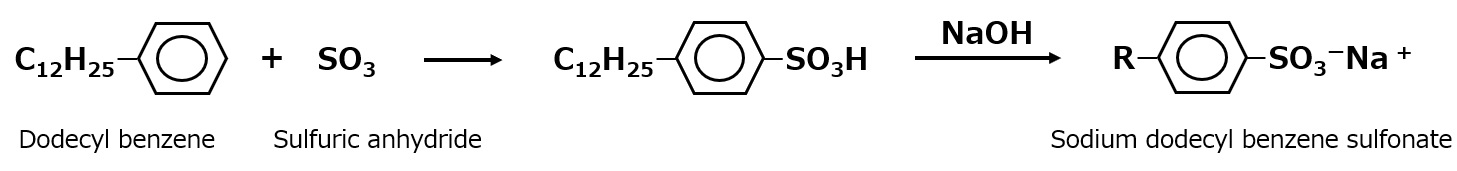
Fig. Synthetic route of sodium dodecylbenzenesulfonate
Sodium alkylbenzenesulfonate Applications and Features
Sodium alkylbenzenesulfonate is easily synthesized, so it is industrially manufactured to a high degree of purity. Commercial products for industrial use are supplied as a light yellow paste with an active ingredient of about 60%, as well as a powdered product that is added sodium sulfate and dried in small granules using a spray dryer.
However, it is household synthetic detergents that are consumed in large quantities, and many synthetic powdered detergents for electric washing machines used in your homes have sodium alkyl benzene sulfonate as their main ingredient.
Sodium alkylbenzenesulfonate is much more soluble in water than soap. Its aqueous solution foams well, producing many more fine bubbles than soap, but its low viscosity makes it easier to dissipate. It has excellent penetrating and cleaning power, and often performs better than sulfates, but it is not necessarily superior in every respect, and has its advantages and disadvantages.
Alkylbenzene Classification and Production Methods
There are various methods for producing alkylbenzene, the raw material for sodium alkylbenzenesulfonate. Through many years of research, it is known that the best alkyl group for alkylbenzene is about a dodecyl group (C12H25-). Therefore, as in the aforementioned example, dodecylbenzene is widely used as a raw material for household detergents. However, even a single bite of dodecyl group can make a big difference depending on the presence or absence of branching.
Among surfactants, the following is a brief introduction to the differences among them, since they are produced in large quantities as the main ingredients in household detergents.
Alkylbenzenes can be divided into two major groups according to the presence or absence of alkyl group branching as follows.
Branched alkyl benzene
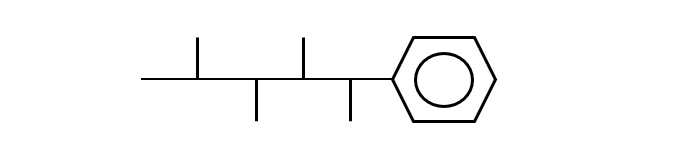
Fig. Branched alkylbenzene
It is synthesized by polymerizing propylene to form propylene tetramer (tetramer) and reacting it with benzene. Branched dodecylbenzene is a typical product.
It is synthesized by polymerizing propylene to form propylene tetramer (tetramer) and reacting it with benzene. Branched dodecylbenzene is a typical product.
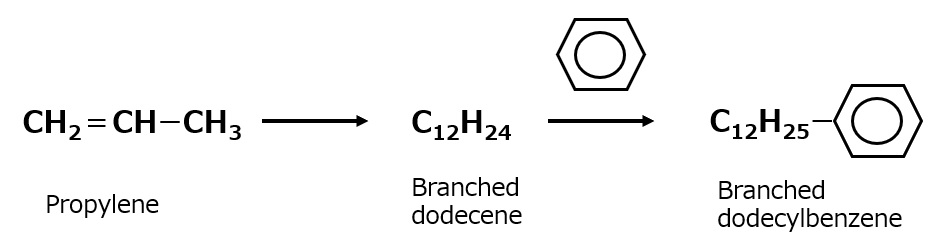
Fig. Synthetic route to branched dodecylbenzene
With the development of petrochemistry, branched alkylbenzenes came to be produced in large quantities at low cost worldwide and became popular as the main ingredient in household washing machine detergents, but their poor biodegradability became a problem and they were replaced by linear alkylbenzenes.
Branched sodium dodecylbenzenesulfonate is abbreviated as ABS (alkylbenzenesulfonate), branched ABS, or hard ABS.
ABS is originally an abbreviation for alkylbenzenesulfonate, but since branched ABS was in its infancy, it is often used to refer only to branched ABS. As for the type of salt, it is sometimes used to refer to sodium salts, since sodium salts are produced in large quantities.
Linear alkyl benzene
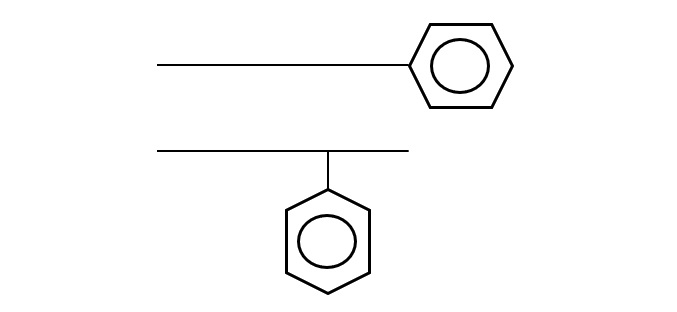
Fig. Linear alkylbenzene
This variety has emerged as an alternative to branched dodecylbenzene as a detergent raw material with better biodegradability. Linear-chain sodium alkylbenzenesulfonate is often abbreviated as LAS (linearalkylbenzenesulfonate), linear chain ABS, or soft ABS.
Linear alkylbenzenes are synthesized by reacting chlorinated paraffins or olefins with benzene.
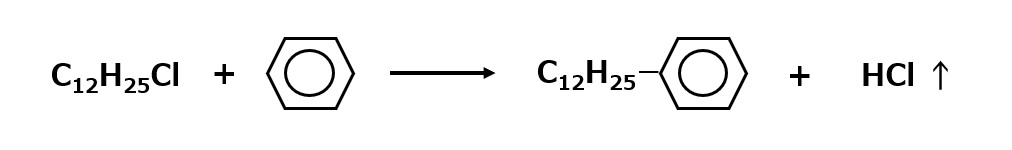
Fig.Synthetic route to linear alkylbenzenes
Chlorinated paraffins and olefins are mixtures of different chain lengths, so they are not pure dodecylbenzenes but mixtures of alkylbenzenes with carbon numbers around 12.
These two types of alkylbenzenes, branched and linear, are equally useful as raw materials for sodium alkylbenzenesulfonate. They are almost equally useful in terms of performance, with one major difference. That is biodegradability.
Biodegradability of alkylbenzenesulfonates
Biodegradability refers to whether the soap is easily or easily degraded by microorganisms in sewage or rivers.
Since soap is made from animal- and plant-derived materials, there is no problem with its biodegradation, since it is quickly decomposed by microorganisms even if it flows into sewage or rivers. However, sodium branched alkylbenzenesulfonate, which was used in large quantities from the 1950s to the 1970s as the main ingredient in electric washing machine detergents, is also resistant to decomposition and foams well even at low concentrations, causing foaming in rivers and sewage treatment plants, which has become a major problem. This has caused foaming in rivers and sewage treatment plants, resulting in a major problem.
The use of branched sodium alkylbenzenesulfonate was thus considered problematic, and linear sodium alkylbenzenesulfonate, which is more biodegradable, came to dominate the mainstream. Sodium higher alcohol sulfates and higher alcohol EO adducts, which are also biodegradable, have also become important to the problem.
Oil-soluble alkylbenzenesulfonates
Sodium alkylbenzenesulfonate, introduced in the previous section, is a water-soluble type of alkylbenzenesulfonate that is consumed in large quantities, especially as a cleaning agent. In this section, another type of alkylbenzenesulfonate, the oil-soluble one, is presented.
Oil-soluble alkylbenzenesulfonates are literally soluble in petroleum and organic solvents.
To make an oil-soluble type, the hydrophobic (lipophilic) alkyl group must be specially enlarged, or a less water-soluble calcium salt must be used instead of a sodium salt.
ABS (Na salt) with large hydrophobic group
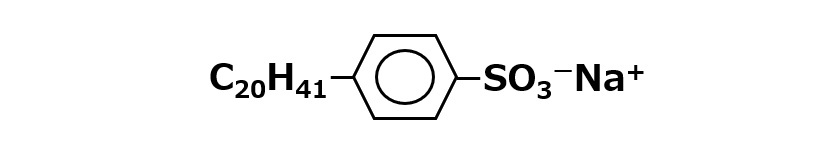
Fig. An example of ABS with a large hydrophobic group portion
Alkylbenzenes with alkyl groups of about 20 carbons are obtained from natural petroleum fractions or by synthesis to produce oil-soluble sodium alkylbenzenesulfonate.
This type of oil-soluble ABS is widely used as a raw material for dry cleaning detergents (charge soap) and as an emulsifier component for mineral oils such as cutting oils. It is characterized by its oil solubility and strong hydrophilic properties.
Alkaline earth metal salts of ABS
Even ordinary ABS, i.e., alkylbenzenesulfonates with alkyl groups of about C12 for detergents, are oil-soluble if they are made less water-soluble, such as calcium salts, instead of sodium salts, and are used as pesticide emulsifier ingredients, for example.
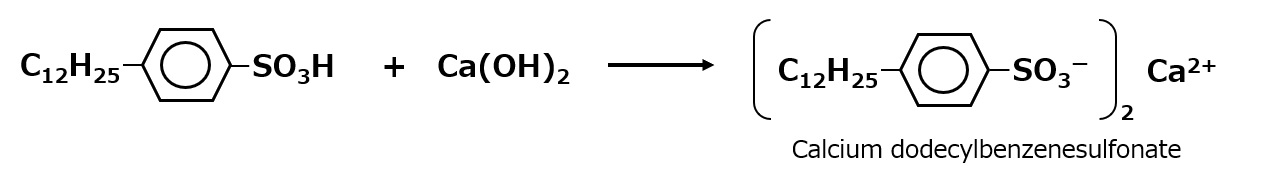
Fig. Synthetic route of calcium dodecylbenzenesulfonate
Alkaline earth metal salts of ABS with a large hydrophobic group portion
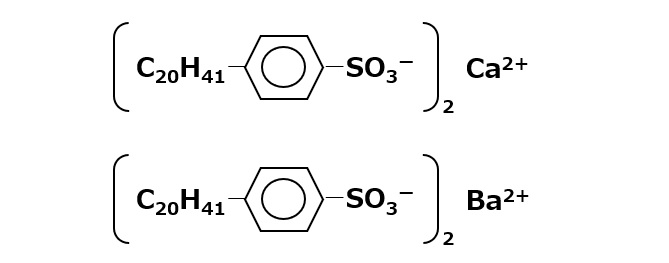
Fig. Alkali earth metal salts of ABS
Calcium alkylbenzenesulfonate or barium alkylbenzenesulfonate, which have alkyl groups of about 20 carbons, are useful as rust inhibitors or heavy oil sludge dispersants.
In addition, sulfonates containing extra Ca(OH)2 or Mg(OH)2 (over-based) are also produced by using a special device when synthesizing these alkali earth metal salts.
These are called over-based calcium alkylbenzene or magnesium alkylbenzene, and are important oil-soluble surfactants as clean dispersants, one of the lubricant additives.
α-olefin sulfonate
When α-olefin is reacted with anhydrous sulfuric acid (SO3), α-olefin sulfonate is obtained as a result of a complex reaction. α-olefin sulfonate is used as a raw material for household detergents. α-olefin sulfonate is also abbreviated as AOS (α-olefinsulfonate).

Fig. Synthetic route of α-olefin sulfonate
Alkanoylmethyltauride
Among alkanoyl methyltaurides, the most famous product is "IGEPON T," developed by IG in Germany. This is a special type of surfactant made by reacting oleic acid chloride with N-methyl taurine, and is also produced in Japan.
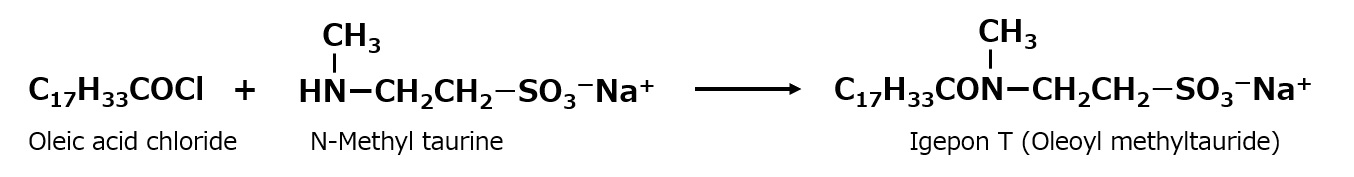
Fig. Synthetic route of oleoylmethyltauride
The amide bond between the hydrophobic and hydrophilic groups is a unique feature of this product. The methyl group attached to the amide group is not an accidental inclusion, but the result of a great deal of research. This product occupies a unique position as a dyeing aid and scouring agent, and is highly valued for its excellent washing texture.
Those in which oleic acid is replaced with palm oil fatty acid are hypoallergenic and are used as detergent ingredients in shampoos. Other known products include those using beef tallow fatty acids, and those in which the methyl group at the amide bond is replaced with a phenyl group.
Dialkyl sulfoammonate
Among the dialkylsulfoammonates, the most famous is Aerosol OT, which was invented in the United States.
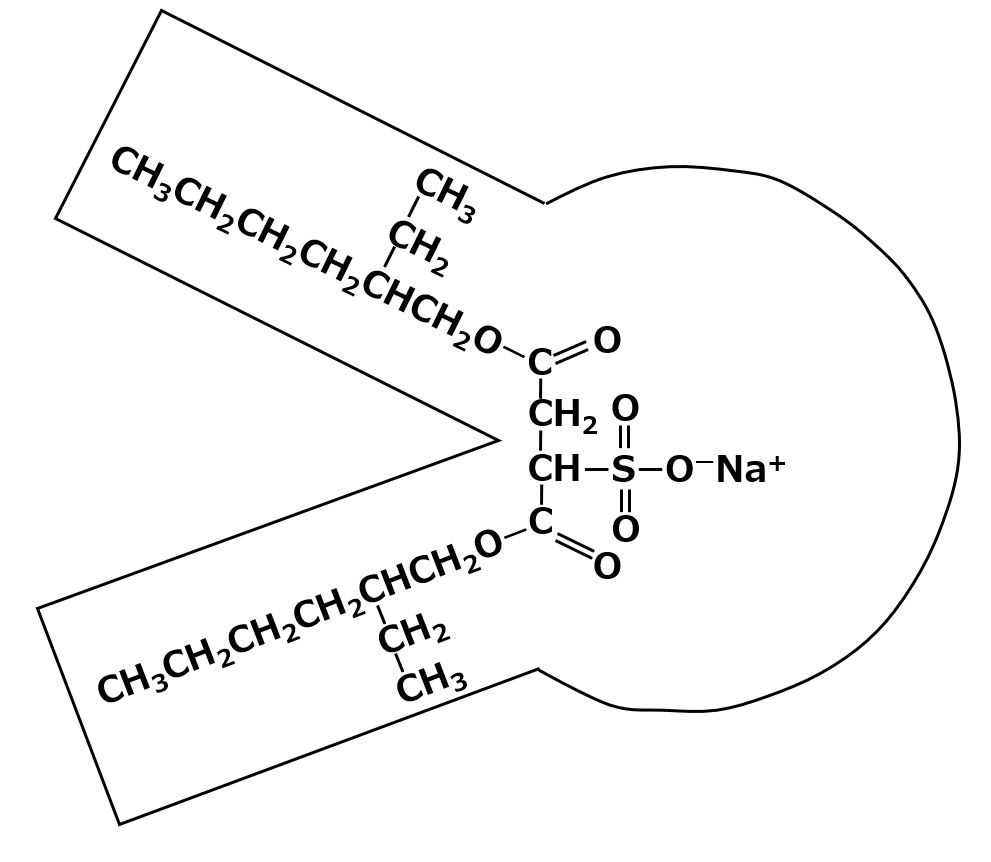
Fig. Dialkyl sulfoammonate (aerosol OT)
Aerosol OT is a sulfonic acid type surfactant of an unusual shape with two branched hydrophobic groups, as shown in the left figure.
The chemical name for this compound is sodium di-2-ethylhexyl sulfoammonate. Please take a close look at the shape of the molecule in the figure on the left. At the base of the branching is -SO3Na+ . In other words, a hydrophilic group is attached to the center of a hydrophobic group.
This compound is such a high performance penetrant that it is hard to imagine a better anionic surfactant. However, its molecular shape means that little detergency can be expected from it, and its main use is as a penetrating agent, with some other uses as an emulsifier, so the amount used itself is not very large.
Also, this type of product has ester bonds in the molecule, which are easily hydrolyzed by strong acids or alkalis, and this is another reason for its limited use.
There are many manufacturers in Japan producing products identical to this one. The synthetic method is completely different from the sulfonates described so far. That is, a diester is made from 2-ethylhexanol and maleic anhydride (or fumaric acid), which is heated while stirred with an acidic sodium sulfite solution to insert a sulfonic group into the double bond of the maleic acid (or fumaric acid).
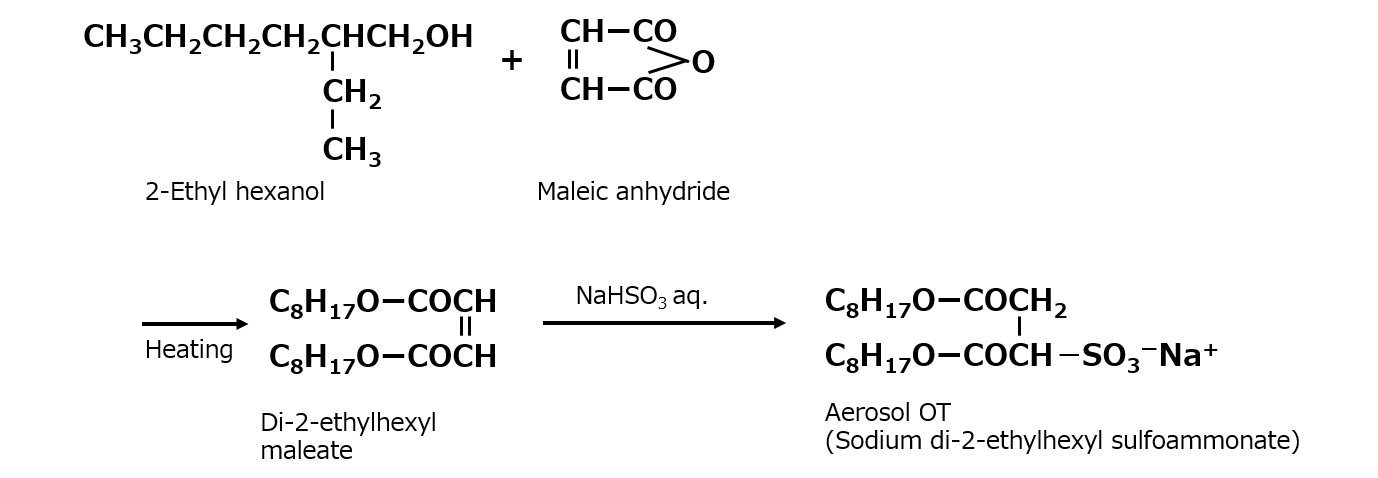
Fig. Sodium di-2-ethylhexyl sulfoammonate
Double bonds such as oleic acid cannot be easily added to acidic sodium sulfite in this way. I won't go into the chemistry, but such reactions are more likely to occur with the double bonds of special compounds such as maleic acid esters.
Sodium alkyl arylsulfoammonate
This sulfation method is used to produce sodium alkyl allyl sulfosulfamate.
This compound is an asymmetric diester with an alkyl group on one side of the diester and an allyl group with a double bond on the other. This structure makes it a reactive surfactant with copolymerizability with radical polymerizable monomers, etc., and it is used as an emulsifier for soap-free emulsion polymerization.
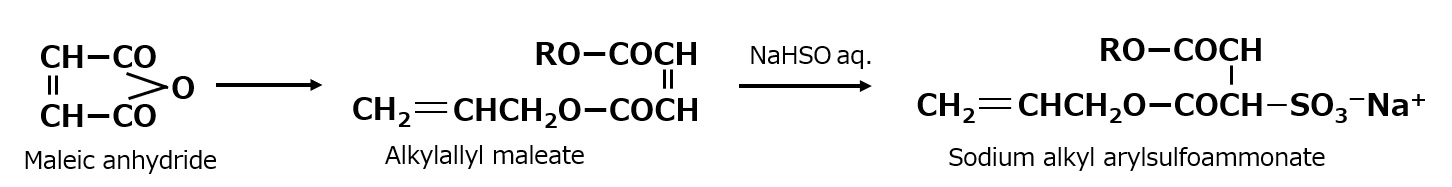
Fig. Synthetic route to sodium alkyl arylsulfosuccinate
Carboxylic acid/sulfonate
The dialkylsulfoammonate ester salts mentioned earlier are obtained by sulfonation of maleic acid diesters and have one sulfonate in the molecule as a hydrophilic group.
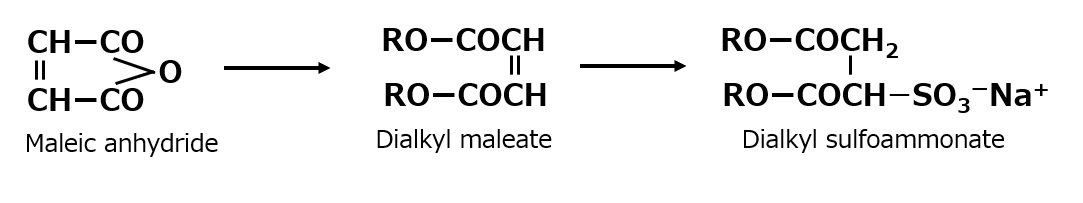
Fig. Dialkyl sulfoammonates
Maleic acid monoesters can be easily obtained from maleic acid by selecting the esterification conditions. Sulfonation of the maleic acid monoester, as in the above reaction, yields alkyl sulfosulfamate disulfonates, each having one sulfonate and one carboxylate. This type of surfactant is hypoallergenic and is used in hair and body shampoos.
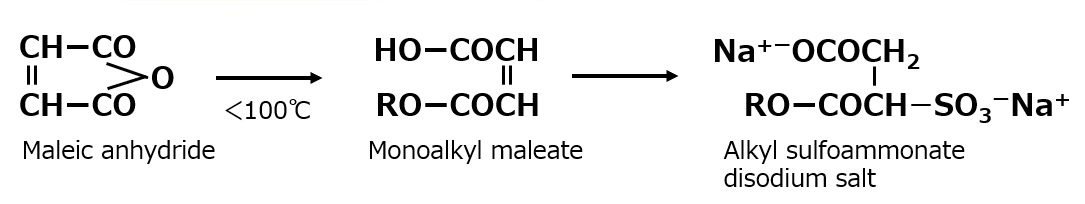
Fig. Alkyl sulfoammonate disodium salt
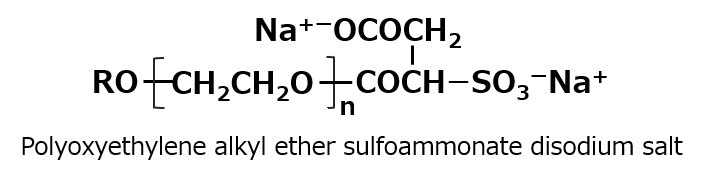
Fig. Polyoxyethylene alkyl ether sulfoammonate disodium salt
In addition, by monoesterification of maleic anhydride with a higher alcohol EO adduct instead of higher alcohol and sulfonation in the same manner, surfactants with carboxylate, sulfonate, and polyethylene glycol chains in the molecule as hydrophilic groups can be obtained. This type of surfactant has excellent foaming properties.
All of these surfactants, like the dialkyl sulfosulphonate ester salts mentioned earlier, have intramolecular ester bonds and are easily hydrolyzed, so they must be used in the neutral to slightly acidic pH range.
Sulfate
Sulfation includes sulfonation and sulfate esterification, and here we discuss sulfate salts obtained by sulfate esterification. Two molecules of sodium hydroxide are required to neutralize one molecule of sulfuric acid, a dibasic acid.
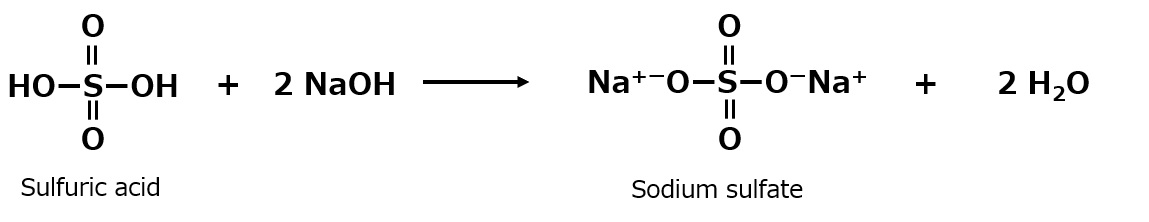
Fig. Neutralization reaction of sulfuric acid (2 equivalents of sodium hydroxide)
If there is only one molecule of sodium hydroxide, the sulfuric acid is only half neutralized to sodium hydrogen sulfate (acidic sodium sulfate). This is also a stable compound.
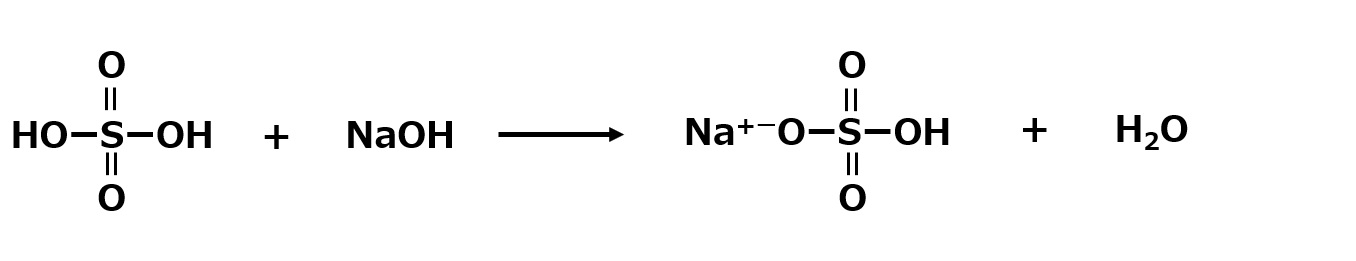
Fig. Neutralization reaction of sulfuric acid (1 equivalent of sodium hydroxide)
Similarly, when making esters from sulfuric acid and alcohol, monoesters and diesters can be made. Taking methanol as an example, we see the following.
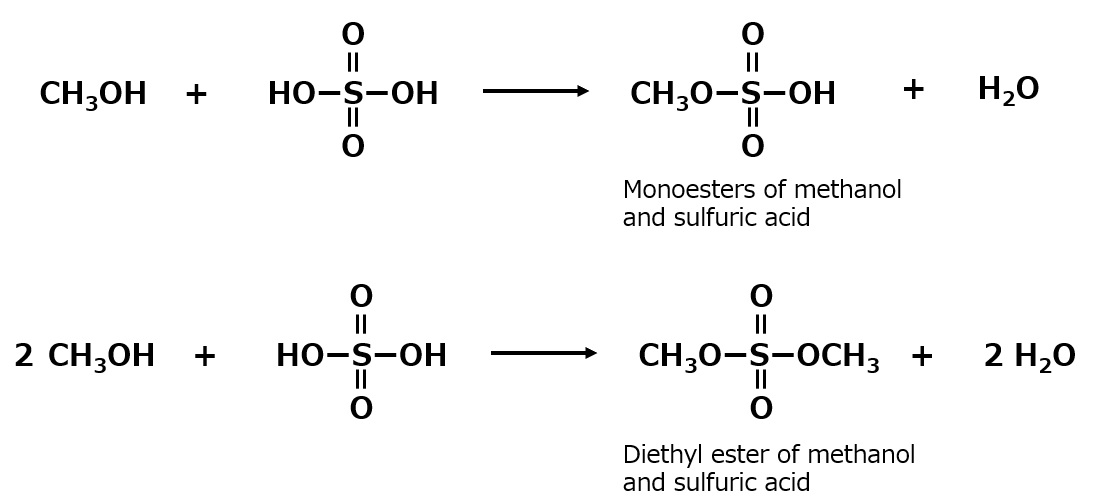
Fig. Esterification reaction of methanol and sulfuric acid
Diesters are insoluble in water, but monoesters are soluble in water. However, if the monoester is dissolved in water, it will gradually hydrolyze to the original methanol and sulfuric acid. To prevent this, neutralize the monoesters with sodium hydroxide to make them stable and neutral in aqueous solution.
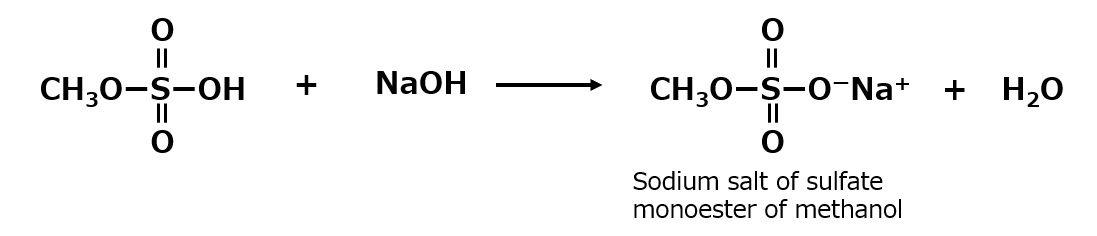
Fig. Neutralization reaction of sulfate monoester of methanol
By utilizing the properties of sulfuric acid, a higher alcohol can be used in place of methanol to produce anionic surfactants.
Furthermore, by using this reaction, most alcohol-like compounds with hydroxyl groups can be converted to sulfates, making it possible to produce a variety of surfactants.
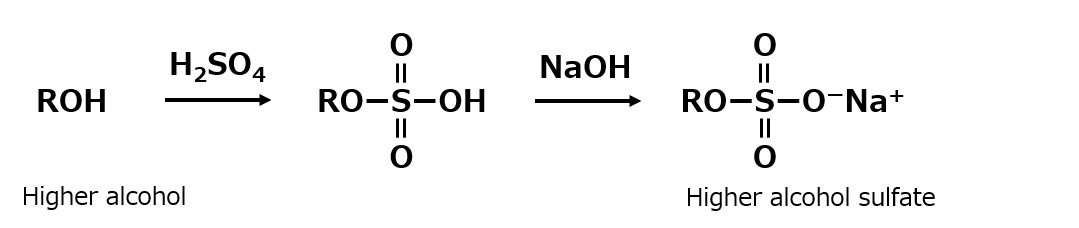
Fig. Synthetic route to higher alcohol sulfates
Sulfation of carbon-carbon double bonds
In addition to alcoholic hydroxyl groups, carbon-carbon double bonds (>C=C<) also react with sulfuric acid to form sulfate monoesters. Therefore, this reaction can also be used to make sulfate ester type surfactants.
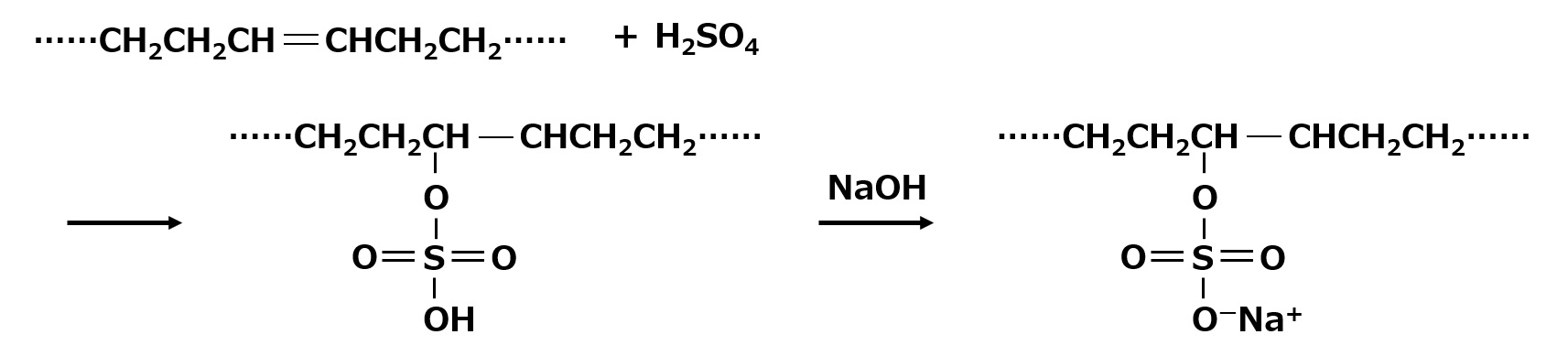
Fig. Sulfate esterification and neutralization of carbon-carbon double bonds
Higher alcohol sulfates (higher alkyl sulfates)
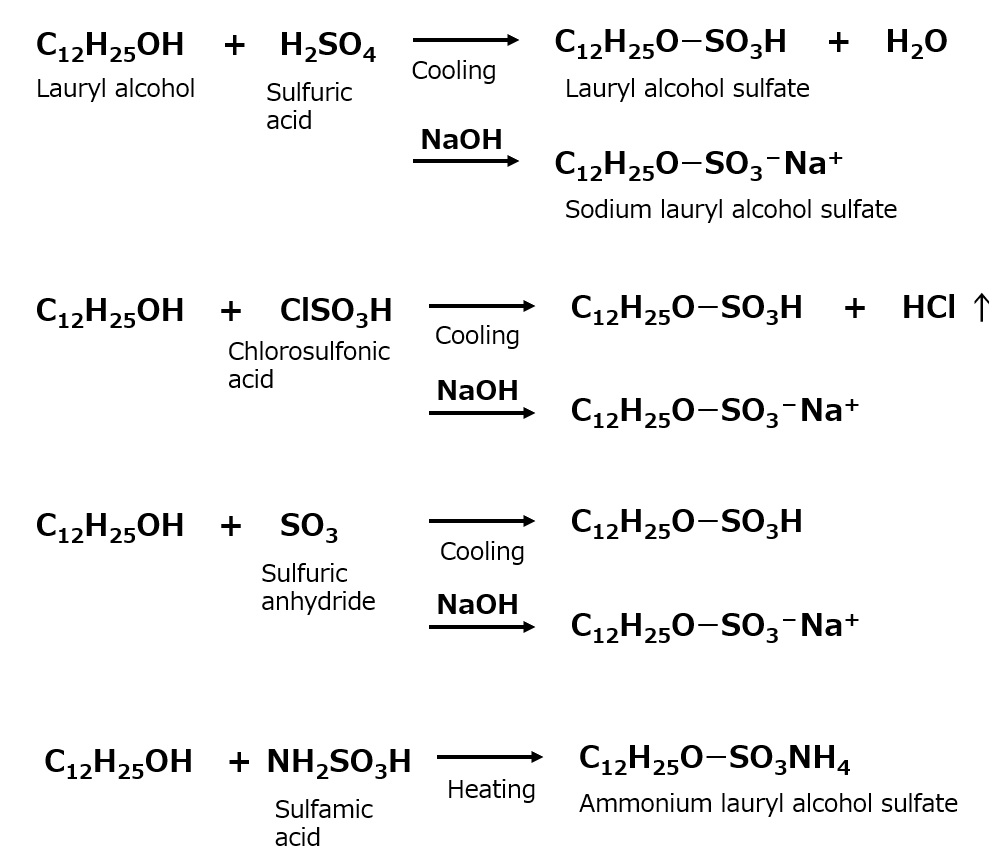
Fig, Sulfation reaction of higher alcohols
An anionic surfactant made by sulfating higher alcohols with long-chain alkyl groups. As in the case of soap, the most suitable carbon number of higher alcohol is 12-18.
Sulfating agents used include sulfuric acid, fuming sulfuric acid, chlorosulfonic acid, and anhydrous sulfuric acid. Sulfamic acid is sometimes used to directly produce ammonium salts.
As already mentioned, there are various types of higher alcohols, each with its own advantages and disadvantages. The most significant difference is the difference in the feel of the detergent made from them. For example, the feel of shampooed hair is smoothest when shampoo made from linear primary alcohols is used.
Sodium sulfate of higher alcohols
| Alcohol | Sulfate | |
|---|---|---|
Lauryl alcohol: C12H25OH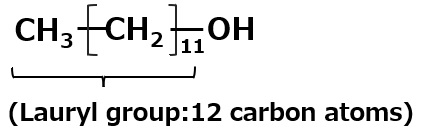 |
→ | C12H25O-SO3- Na+ Sodium lauryl alcohol sulfate |
Cetyl alcohol: C16H33OH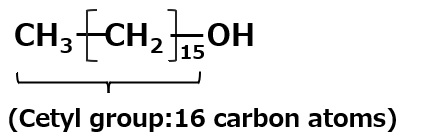 |
→ | C16H33O-SO3- Na+ Sodium cetyl alcohol sulfate |
Stearyl alcohol: C18H37OH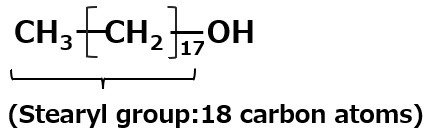 |
→ | C18H37O-SO3- Na+ Sodium stearyl alcohol sulfate |
Oleyl alcohol: C18H35OH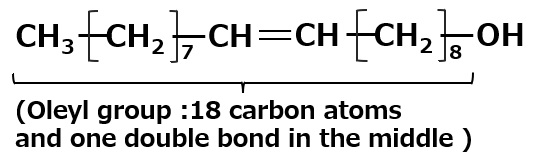 |
→ | C18H35O-SO3- Na+ Sodium oleyl alcohol sulfate |
The relationship between the number of carbons (chain length) of the alkyl group of the raw material alcohol and the water solubility or detergency of its sulfate ester salt is similar to that of soap. Sodium lauryl alcohol sulfate, which has a short chain length (C12), is relatively hydrophobic and soluble in water even at low temperatures and has good detergency. Sodium stearyl alcohol sulfate, which has a long chain length (C18), is not soluble in water, and its water solubility and detergency are not high unless the water is hot, making it difficult to use alone.
Sodium cetyl alcohol sulfate, which has a medium chain length (C16), is between lauryl and stearyl and is somewhat similar to stearyl. Sodium oleyl alcohol sulfate, however, has a weakly hydrophilic double bond in the middle of its molecule, which gives it superior water solubility and detergency, even though the carbon number is 18. This double bond action is exactly the same as in the case of soap, and it is present throughout the entire surfactant.
Bound Sulfate Content and Total Fat Content
Here, we discuss the amount of bound sulfate and total fat content, which are necessary to understand sulfates as surfactants.
To make the story more concrete, let us take sodium lauryl alcohol sulfate in the following product form as an example.
| Main ingredient | C12H25OSO3-Na+ | 90.0% |
| Unreacted product | C12H25OH | 4.0% |
| By-product | Na2SO4 | 1.0% |
| Water | H2O | 5.0% |
Calculation of bound sulfuric acid content
The amount of bound sulfate is a numerical value that indicates how much sulfate is bound to the higher alcohol (in this case, lauryl alcohol) as SO3 in the product. We have often mentioned that water solubility changes depending on whether the hydrophobic group per hydrophilic group is long or short. Therefore, the expression of the amount of bound sulfuric acid is the opposite of the hydrophobic group lengths described so far, but the concept is the same.
Thus, for this product,
Molecular weight of C12H25OSO3-Na+ = 288.4
Molecular weight of SO3 = 80.1, and content of main component = 90.0%, then
The amount of bound sulfuric acid (%, per product) = 80.1/288.4 × 100 × 0.900 = 25.0.
In practice, the amount of combined sulfuric acid (%) is measured and the main component content (%) is obtained by inverse calculation of the above formula. The measurement method is omitted.
Total Fat Calculation
Total fat content is a number that indicates what percentage of oil is contained in the product.
Thus, for this product, if the molecular weight of C12H25OH = 186.3,
Fatty derived from main ingredients (%) = 186.3/288.4 × 100 × 0.900 = 58.1
Fat derived from unreacted material (%) = 4.0
Total fat (%, per product) = 58.1 + 4.0 = 62.1
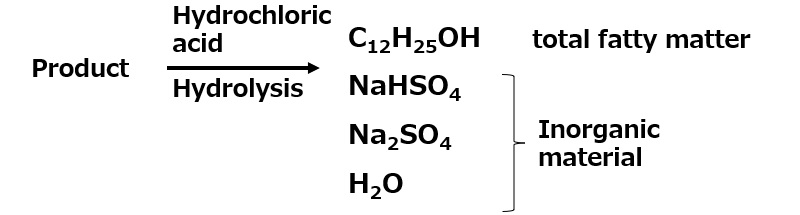
Fig. Reaction during measurement of total fat and bound sulfuric acid content
To measure the actual total fat content (%), the product is heated with hydrochloric acid to remove the sulfate groups that are bound to it, and then it is converted to the form of higher alcohol before sulfation, and the amount is extracted with diethyl ether or the like.
Calculation of main and unreacted content using bound sulfate and total fat content
In the previous section, we discussed total fat and bound sulfate content.
So what would you know if you saw the following label for sodium cetyl alcohol sulfate?
| Total fat | 40.0% |
| Bound Sulfate/Total Fat | 28.0% |
i) Sodium cetyl alcohol sulfate content (% of main ingredient)
Substituting 40.0% total fat content into the formula Bound Sulfate Content/Total Fat Content × 100 = 28.0 (%), we obtain Bound Sulfate Content = 11.2%.
Substituting the molecular weight (344.5) of sodium cetyl alcohol sulfate (C16H33OSO3- Na+ , hereafter referred to as "main ingredient") into the formula below, the content of main ingredient is calculated to be 48.2%.
Amount of bound sulfate (%) = Molecular weight of SO3 (80.1) / Molecular weight of sulfate ester salt x Sulfate ester salt content (%)
ii) Reaction rate of cetyl alcohol
Substituting the molecular weight of cetyl alcohol (242.4), the content of the main ingredient (48.2%) and the molecular weight of the main ingredient (344.5) into the formula below, the fat content derived from the main ingredient is calculated to be 33.9%.
Fatty matter derived from main ingredient (%)
= molecular weight of cetyl alcohol/molecular weight of main ingredient × content of main ingredient (%)
= 242.4/344.5 × 48.2 = 33.9
From these values, the reaction rate of cetyl alcohol and also the unreacted fatty (unreacted cetyl alcohol) content can be calculated.
Reaction rate (%)
= fat derived from main ingredient (%) / total fat (%) × 100
= 33.9/40.0 × 100 = 84.8
Fat derived from unreacted material (unreacted cetyl alcohol, %) = total fat (%) - fat derived from main ingredient (%) = 40.0-33.9 = 6.1
This means that 6.1% of unreacted cetyl alcohol remains.
As described above, the content of main ingredients and unreacted materials can be determined from the total fat content and the amount of bound sulfate/total fat content, so it is important to handle surfactants with these values in mind. Now, the bound sulfate content/total fat content of pure sulfate is as shown in the table below.
As can be seen in the table, the longer the hydrophobic group, the less hydrophilic the theoretical value of bound sulfate/total fat (%) becomes. However, the theoretical values for stearyl alcohol and oleyl alcohol are almost the same, but the actual water solubility is significantly different. This is because the weak hydrophilicity due to the double bond has nothing to do with the theoretical value, and even if the theoretical value is almost the same, oleyl is more soluble in water because of the double bond.
Therefore, hydrophilicity and hydrophobicity, such as double bonds and ester bonds, which have little to do with the amount of bound sulfuric acid, hardly appear as numbers in this method. In general, the hydrophilicity of the sulfate ester salt can be quickly estimated by knowing the amount of bound sulfate as well as the type of alcohol or fat used as the raw material.
Theoretical value of higher alcohol sulfate
| Base alcohol | Sulfate | Bound Sulfate Content/ Total fat (%) |
|---|---|---|
| Lauryl alcohol | C12H25OSO3-Na+ | 43.0 |
| Myristyl alcohol | C14H29OSO3-Na+ | 37.4 |
| Cetyl alcohol | C16H33OSO3-Na+ | 33.0 |
| Stearyl alcohol | C18H37OSO3-Na+ | 29.6 |
| Oleyl alcohol | C18H35OSO3-Na+ | 29.8* |
The values shown in this table are those when all the higher alcohols in the raw material are sulfated. In industrial terms, it is difficult to achieve 100% reaction, and the product is not manufactured except in special cases. As calculated earlier, usually 80% or 90% of the theoretical value is produced. However, since not all reacted products have better performance, the reaction rate is researched and manufactured according to the purpose of each product. For this reason, even if the same sodium sulfate ester of higher alcohols is used, each company's product has various characteristics, and they are by no means identical.
In the table above, the theoretical value of the amount of bound sulfate/total fat (%) of oleyl alcohol is stated as 29.8%, but this is when only its hydroxyl group reacts, and in reality the true theoretical value is about 29.8 x 2 = 59.6%, since the double bond in the molecule also becomes sulfate. In reality, however, products with such high levels of combined sulfates are not manufactured. In many products, only the hydroxyl group is sulfated, only the double bond is sulfated, and in some cases, both groups are sulfated.
Therefore, for oleyl alcohol sulfated products, the theoretical value of bound sulfate/total fat (%) can be 29.8% or higher, which can be interpreted as a high ratio of both double bonds and hydroxyl groups being sulfated. However, those that react with both have good water solubility, but not much detergency.
These higher alcohol sulfates are superior to soap in both solubility and detergency, and have various advantages, such as being neutral in aqueous solution and not damaging wool, etc. Moreover, they do not precipitate like soap when used in hard water, so they are widely used in both industrial and household applications. However, this type of soap also has its drawbacks: when the aqueous solution becomes highly acidic, it is hydrolyzed back to its original form of higher alcohol, and it also decomposes easily when exposed to high temperatures.
Higher alcohol ethylene oxide adduct sulfates (higher alkyl ether sulfates)
Higher alcohol EO adduct sulfates are similar to the higher alcohol sulfates described in the previous section.
In other words, ethylene oxide is added to higher alcohols, which are then converted to sulfates.

Fig. Sodium Lauryl Alcohol EO Adduct Sulfate
Higher alcohol EO adducts are called higher alkyl polyethylene glycol ethers, or higher alkyl ethers for short, so higher alcohol EO adduct sulfates are sometimes called higher alkyl ether sulfates.
The above formula describes the most commonly used sodium lauryl alcohol EO adduct sulfate. The number of moles of ethylene oxide added (n) is usually between 2 and 4.
Properties and Applications of Lauryl Alcohol EO Adduct Sulfates
What is the difference between lauryl alcohol sulfate and lauryl alcohol EO adduct sulfate? When polyethylene glycol chains are added, water solubility is improved and foaming characteristics in hard water are enhanced.
Therefore, higher alcohol EO adduct sulfates are widely used as base agents for shampoos.
Higher alcohol sulfates are known to cause skin irritation. Higher alcohol EO adduct sulfates have a distribution in the number of moles of EO adduct, and include a small amount of higher alcohol sulfates to which ethylene oxide is not added. In recent years, it has become possible to synthesize higher alcohol EO adducts with fewer unreacted alcohols by adding ethylene oxide to alcohols using a special catalyst, and commercially available low-irritant higher alcohol EO adduct sulfates with reduced higher alcohol sulfate content. In this field, too, the advancement of synthetic alcohols has been promoted.
In this field as well, synthetic alcohols have made great inroads, and oxo alcohol and Zieger alcohol are widely used in shampoos and other products, as are natural lauryl alcohols.
EO adduct sulfates of secondary alcohols
Secondary alcohols (secondary alcohols), which are synthetic alcohols obtained by air oxidation of paraffin, are also used in liquid detergents as higher alcohol EO adduct sulfates. Due to the convenience of synthesis, these secondary alcohols are commercially available as 3-mole ethylene oxide adducts with C11~C15 alkyl group carbons under trade names such as Tergitol and Softanol.
Sulfated oils, sulfated fatty acid esters and sulfated fatty acids
In the previous section, we explained that the hydroxyl groups and double bonds of higher alcohols react with sulfuric acid to form sulfate esters, which are surfactants. Now, what about fatty acids that also have hydroxyl groups and double bonds, or esters of such fatty acids?
As expected, sulfation produces sulfate ester type anionic surfactants. Typical fatty acids and their esters used in sulfation are listed in the table below.
Raw materials containing typical fatty acid esters with hydroxyl groups or double bonds
| Type of fatty acid ester | |||
|---|---|---|---|
| Fats and oils (glycerin triester) |
Low alcohol esters (synthetic) |
||
| Unsaturated fatty acid | Oleic acid | Olive oil, Peanut oil, Beef oil |
Methyl Oleate C17H33COOCH3 Butyl oleate C17H33COOC4H9 |
| Ricinoleic acid | Castor oil | Methyl ricinoleate Butyl ricinoleate |
|
In addition, since the names of many fats and oils will appear in the future, a composition table of each fat and oil is included in the table below to help you understand what fatty acid glycerides each oil is.
When the esters of unsaturated fatty acids shown in the table above are actually sulfated, they have properties very different from those of higher alcohol sulfates. This is because the sulfate groups, which are hydrophilic groups, are attached near the center of the molecule. In this case, it is unlikely that the sulfated products will have the strong detergent power of the higher alcohol sulfates. In fact, most of these sulfated products are used for special textile industry applications other than detergents.
The following is a brief introduction to the different types of hydrophobic group materials.
Fatty acid composition of major fats and oils*.
| Carbon number | Fatty Acids | Coconut oil | Palm kernel oil |
Palm oil | Olive oil | Soybeans oil |
Peanut oil |
Rice bran oil |
Rapesed oil |
Caster oil |
Beef fat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | C8F0 ** (Caprylic acid) |
5.8 | 2.2 | ||||||||
| 10 | C8F0 (Capric acid) |
6.5 | 2.8 | trace | |||||||
| 12 | C12F0 (Lauric acid) |
51.2 | 49.1 | trace | trace | ||||||
| 14 | C14F0 (Mystilic acid) |
17.6 | 15.1 | 1.0~1.1 | trace | 0.2 | 3.3 ~3.5 |
||||
| C14F1 | |||||||||||
| 15 | C15F0 | ||||||||||
| C15F1 | trace | ||||||||||
| 16 | C16F0 (Palmitic acid) |
8.5 | 8.0 | 45.3 | 10.6~ 11.8 |
10.8 ~12.2 |
9.9~ 12.0 |
17.6 | 3.4 | 1.0 ~1.1 |
26.6 ~27.4 |
| C16F1 | trace | trace | trace | ||||||||
| 17 | C17F0 | ||||||||||
| C17F1 | |||||||||||
| 18 | C18F0 (Stearic acid) |
2.7 | 2.4 | 4.3 ~4.4 |
2.2 ~3.6 |
3.4 ~4.2 |
2.1 ~4.2 |
1.3 | 1.2 | 0.7 ~1.0 |
18.2 ~25.8 |
| C18F1 (Oleic acid) |
6.5 | 18.4 | 38.8 ~40.3 |
71.0 ~77.2 |
20.4 ~23.1 |
37.3 ~49.3 |
39.5 | 16.5 | ***** 3.1 ~4.1 |
35.7 ~41.2 |
|
| C18F2 (linoleic acid) |
1.2 | 2.0 | 8.8 ~9.8 |
7.2 ~13.0 |
53.7 ~55.8 |
31.6 ~41.7 |
38.2 | 16.2 | 4.4 ~5.2 |
trace ~3.3 |
|
| C18F3 (linolenic acid) |
trace ~0.1 |
0.9 | 6.4 ~10.1 |
1.0 ~1.8 |
1.5 | **** 9.5 |
0.9 | trace ~1.1 |
|||
| 20 | C20F0 | 0.1 ~0.7 |
1.1 ~1.7 |
0.5 | |||||||
| C20F1 | 0.5 | ||||||||||
| 22 |
C22F0 | 1.9 ~3.5 |
0.2 | 0.7 | |||||||
| C22F1 |
41.4 |
||||||||||
| C24F0 |
0.3 |
||||||||||
| Other | 0.0~2.0 |
Sulphated oil
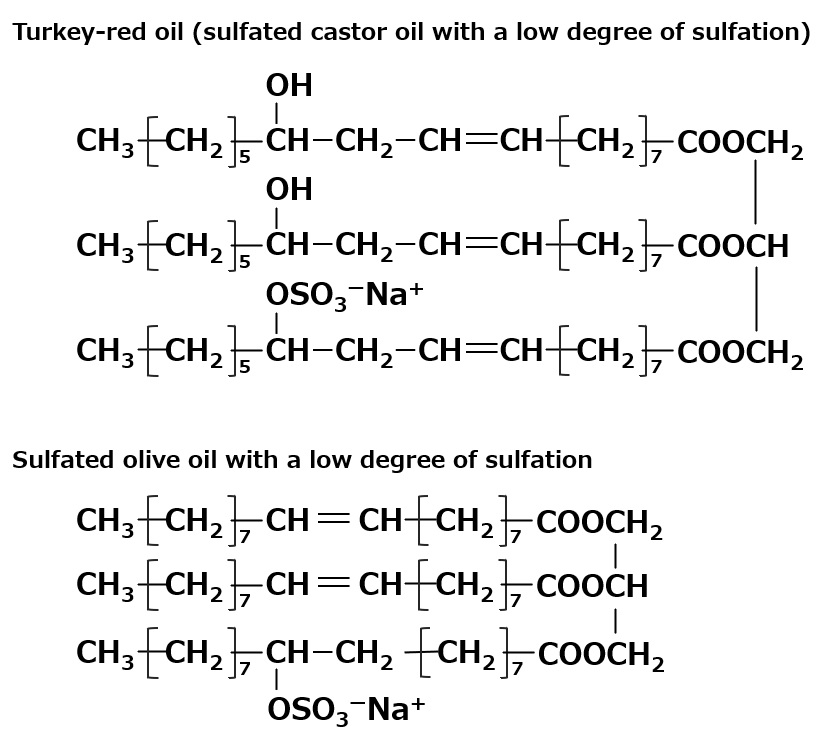
Fig. Sulfated oil
Sulfated oil is a generic term for natural unsaturated fats and oils or unsaturated wax oils that have been directly sulfated and neutralized.
Turkey-red oil is a representative of sulfated oils that have been manufactured for a long time and are now rarely used because of the production of various new surfactants with characteristics that make castor oil suitable for sulfuric acid.
In addition to Turkey-red oil, sulfated beef tallow and sulfated peanut oil are industrially produced as sulfated oils. Since the amount of sulfuric acid bound in these oils is relatively small, they are hydrophilic to the extent that they are barely soluble in water or form emulsions.
Therefore, they are not used as detergents at all. They used to be widely used as base agents for spinning oils, weaving oils, and textile finishing agents, but recently the demand for these products has been decreasing.
Sulfated fatty acid esters
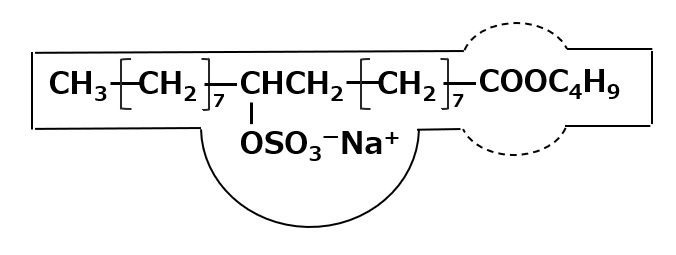
Fig. Sulfated Fatty Acid Esters
Apart from natural oils and fats, sulfate-type anionic surfactants can also be made by sulfating lower alcohol esters of unsaturated fatty acids, such as butyl oleate or butyl ricinoleate.
They all have the performance of roto oil, which can be considered as an improvement of roto oil. They have higher binding sulfate/total fat content (15-20%) than roto oil, better penetrating power, and relatively low foaming, which is why they are used as dye auxiliaries.
Sulfated fatty acids
It is a sulfated product of unsaturated fatty acid as it is, and its properties are similar to those of fatty acid ester sulfides.
Olefin sulfate
We have already mentioned in the previous section that higher alcohols and fats are compounds with hydroxyl groups and double bonds that can be sulfated. These are all obtained from plants and animals. So, can anything like that be obtained from petroleum itself? If we select double-bonded hydrocarbons (olefins) with chain lengths from C12 to C18, we should be able to synthesize good sulfate surfactants. These are collectively called sulfated olefins.
This type of detergent has long been produced mainly in Europe, with Shell's "Teepol" being the most famous. This detergent is made by sulfating C12~C18 alpha-olefins (olefins with a double bond at the end of the molecule) made by breaking down paraffin wax at high temperatures.
As seen in the following equation, sulfuric acid is not attached to the end of the olefin molecule, but to the carbon next to it. α-olefin is not easily sulfated in its entirety at once, so unreacted material is recovered to produce a product with high bound sulfate content.
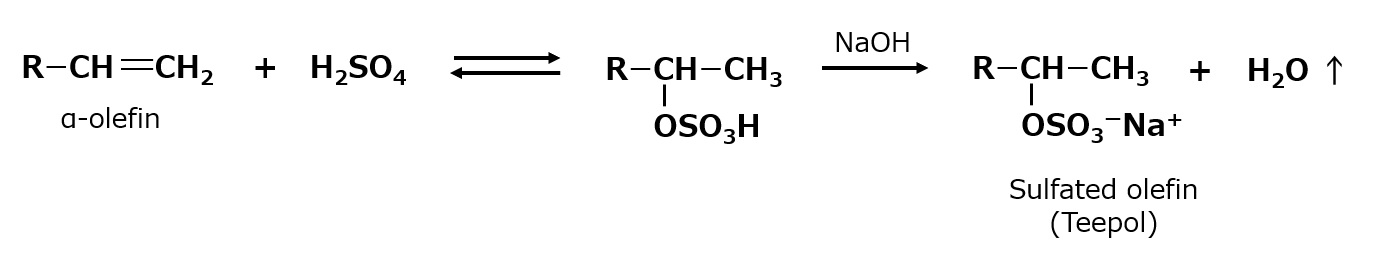
Fig. Synthetic route to sulfated olefins
Teepol dissolves well in water and can be made into a thick solution, and is used as a raw material for liquid detergents. Sulfonation of α-olefin with anhydrous sulfuric acid also produces α-olefin sulfonates in the sulfonate form rather than the sulfate ester form.
Phosphates
Phosphate-type anionic surfactants are mainly used as antistatic agents or emulsifiers for synthetic fibers. Phosphate ester salts of higher alcohols are widely used.
Higher alcohol phosphates (higher alkyl phosphates)
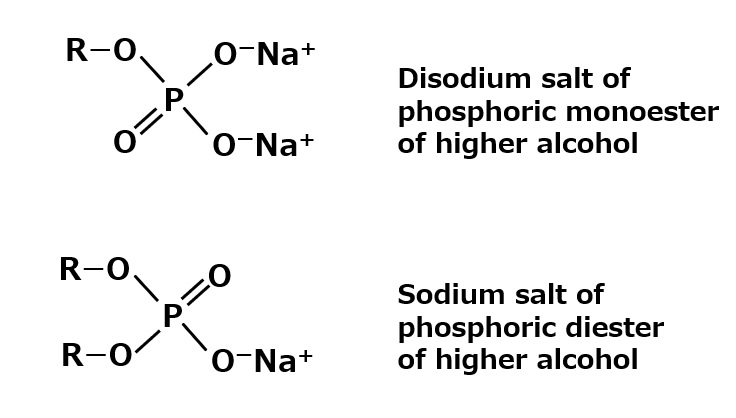
Fig. Sulfated Fatty Acid Esters
The two typical chemical structures are shown in the figure on the left.
The monoester type dissolves well in water, while the diester type is difficult to dissolve and only emulsifies. Many of the products actually used are mixtures of these two types.
Phosphorylation with anhydrous phosphoric acid is often used in the synthesis of phosphate esters. This reaction produces a mixture of monoesters and diesters, the ratio of which can be controlled quite freely by the synthetic conditions eo.
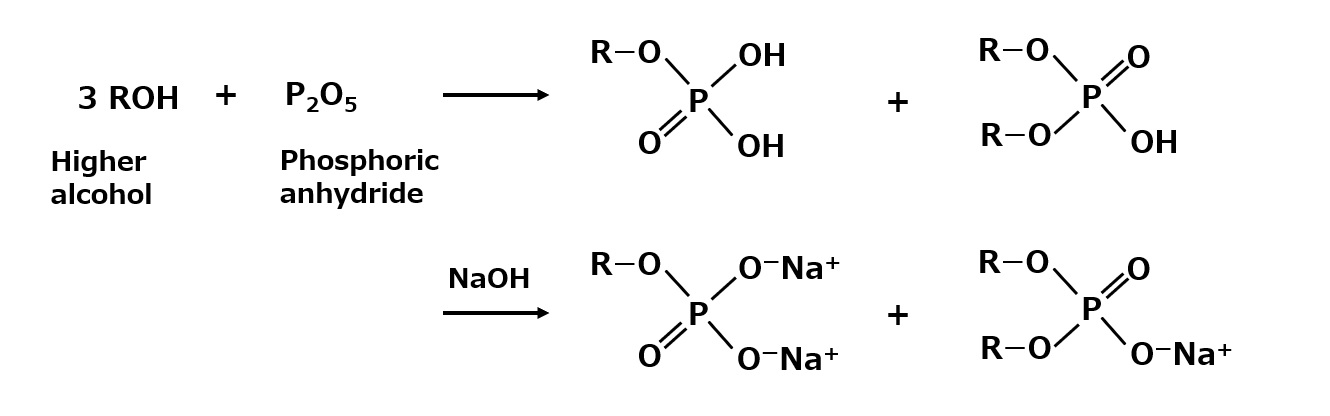
Fig. Synthesis of phosphate salts (phosphorylation with anhydrous phosphoric acid)
Higher alcohol ethylene oxide adduct phosphates (higher alkyl ether phosphates)
The most widely used phosphate ester type surfactants are the phosphate ester salts of higher alcohol EO adducts. This type has good water solubility due to its polyethylene glycol chain, and its antistatic performance is generally superior to that of higher alcohol phosphate salts.
Sodium salt or amine salt forms are used, each with its own unique twist. The triester type in particular is not an anionic surfactant but a nonionic surfactant. They are used for special applications where anionic surfactants are not allowed.
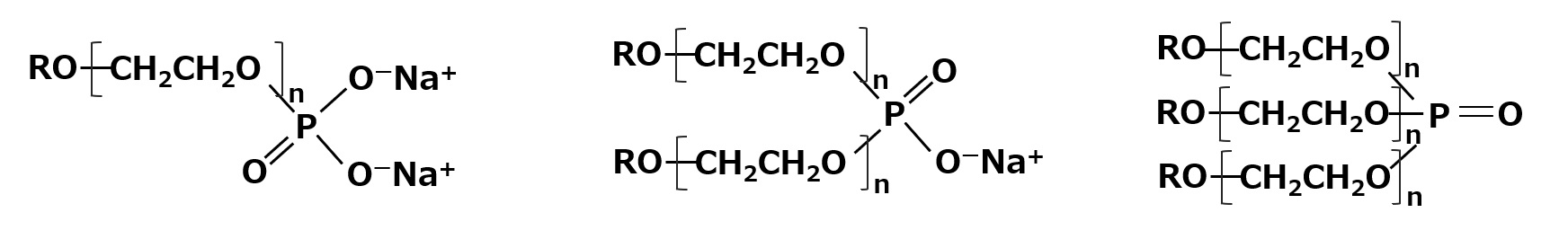
Fig. Phosphate
Synthesis of triesters is often done by reaction with phosphorus oxychloride.
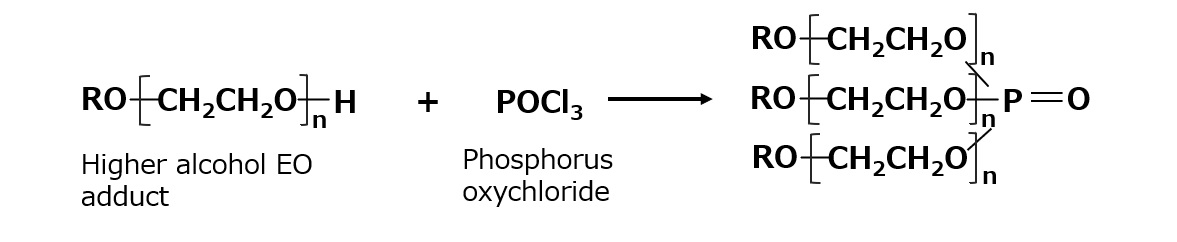
Fig. Synthetic route of phosphate triester
Generally speaking, phosphate surfactants are rarely used alone, but are more often used as ingredients in formulations.
Dithiophosphate
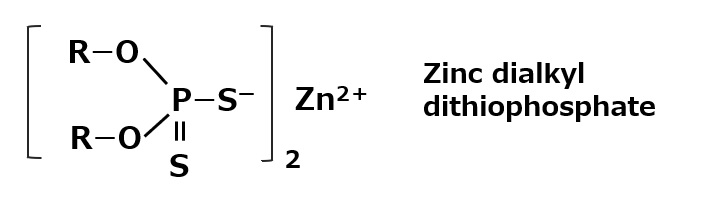
Fig. Zinc dialkyl dithiophosphate
There is a group of oil-soluble anionic surfactants, called zinc dialkyl dithiophosphates, whose chemical structure is similar to that of phosphate salts.
They are well known as a lubricant additive and are widely used as antioxidants and anti-wear agents (extreme pressure additives).
Anionic Surfactant Summary
The above is a rough description of the important anionic surfactants. These are classified by the combination of hydrophobic and hydrophilic groups as shown in the table below.
All but soap have good hard water resistance. Sulfates are relatively stable in alkaline water, but are easily decomposed in acidic water. In addition, those with ester bonds such as -COOCH2- in the molecule are easily decomposed by alkali or acid. We hope you fully understand these points, and we hope this table will help you organize your knowledge so far.
Classification of Anionic Surfactants*
| Calboxylate (salts of fatty acid) -COONa |
Sulfates -OSO3- Na+ |
Sulfonate -SO3- Na+ |
Phosphate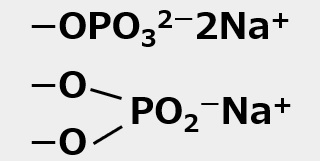 |
Dithiophosphate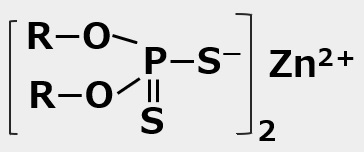 |
|
|---|---|---|---|---|---|
| Paraffin | ー | ー | ** | ー | ー |
| α-olefin | ー | Detergents such as Teepol | α-olefin sulfonates |
ー | ー |
| Higher alcohol | ー | Higher alcohol sulfates (detergents, emulsifiers) |
ー | (Antistatic agents for textiles, etc.) |
Zinc dialkyl dithiophosphates (lubricant additives) |
| Fatty acid | Soaps (detergents, emulsifiers) |
Sulfated fatty acids (dyeing aid) |
α-Sulfonated fatty acids | ー | ー |
| Fatty acid ester | ー | Sulfated fatty acids Ester (dyeing auxiliaries) |
α-Sulfonated fatty acid esters | ー | ー |
| Oils and fats | ー | Sulfated oil (dyeing auxiliaries and textiles lubricants such as turkey-red oil) |
ー | ー | ー |
| Alkyl benzene | ー | ー | Sodium or alkalin earth metal salts of alkyl benzenesulfonate (detergents, emulsifiers, penetrating agents, lubricant additives) |
ー | ー |
 |
ー | ー | Detergents such as Igepon T | ー | ー |
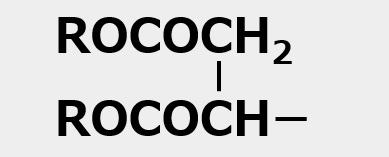 |
ー | ー | Penetrating agents such as Aerosol OT | ー | ー |
Related Products & Topics
Related products

- Anionic surfactant
Sulfosuccinate-type anionic surfactant "SANMORIN OT-70"
Approximately a 70 mass% solution of a sodium dioctyl sulfosuccinate that has particularly excellent penetrating power and surface tension lowering ability among surfactants.

- Cosmetics raw materials
Anionic Surfactant for Cosmetics with Excellent Foaming Properties"BEAULIGHT® SHAA"
"BEAULIGHT® SHAA" has a high foaming speed and forms a fine lather. As a hypoallergenic ingredient, it is suitable as a base material for shampoos, body soaps, etc.

- Cosmetics raw materials
Biodegradable Ether Carboxylic Acid-based Hypoallergenic Detergent Base"BEAULIGHT® LCA-25N"
As a hypoallergenic detergent, "BEAULIGHT® LCA-25N" is suitable for sulfate-free shampoos, body soaps, etc.

- Dispersant
Polycarboxylic acid type pigment dispersant "CARRYBON L-400"
"CARRYBON L-400" has excellent dispersibility in water of various inorganic pigments such as calcium carbonate, clay, satin white, and aluminum hydroxide.

- Antistatic agent
Low Molecular Weight Antistatic Agents "CHEMISTAT"
"CHEMISTAT" can be used for various applications such as kneading into inks, paints, and resins

- Lubricant
- Rust inhibitor
Surfactant-based rust inhibitors (corrosion inhibitors)"SANHIBITOR"
| Link to Sanyo Chemical's corporate website |
|---|
Anionic Surfactant with Excellent Wetting and PermeabilityAnionic Surfactant for cosmetic raw materialsBEAULIGHT ESS Polycarboxylate-Type Dispersant for Inorganic Pigments, Having an Excellent Dispersion StabilityLow molecular weight type antistatic agetnsCHEMISTAT 1100, CHEMISTAT 3033, CHEMISTAT 3500, CHEMISTAT Y-400 Surfactant type rust inhibitorsSANHIBITOR 102, SANHIBITOR 150, SANHIBITOR No.2-1, SANHIBITOR No.50, SANHIBITOR OMA-10 |
Topics



This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.





