Introduction to Cationic Surfactant
What is a surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
Basic structure of a surfactant
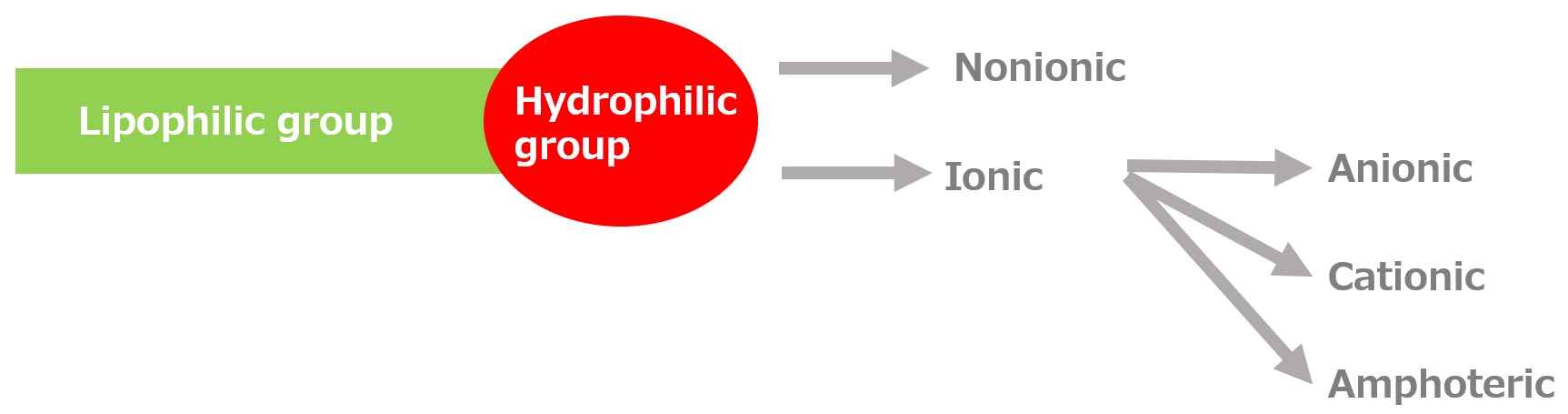
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-loving parts) and hydrophilic groups (water-loving parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant |
-Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH |
-Laundry detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil |
-Polyoxyethylene alkyl ether etc. |
| Anionic surfactant |
-Excellent emulsification and dispersibility -Good lather -Temperature insensitive |
-Laundry detergent -Shampoo -Body soap |
-Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant |
-Adsorption to fibers -Antistatic effect -Bactericidal |
-Hair conditioner and treatment -Fabric softener -Disinfectant |
-Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant |
-Mild on skin -Excellent solubility in water -Synergistic with other active components |
-Shampoo -Body soap -Kitchen detergent |
-Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant functions introduction video
It consists of seven short movies for each function.
0:00 Introduction of surfactants functions
0:16 Part1 Washability
1:00 Part2 Permeability
2:10 Part3 Dispersion
2:55 Part4 Foaming properties
3:25 Part5 Defoaming properties
3:39 Part6 Smoothness
4:20 Part7 Antibacterial properties
What is a cationic surfactant?
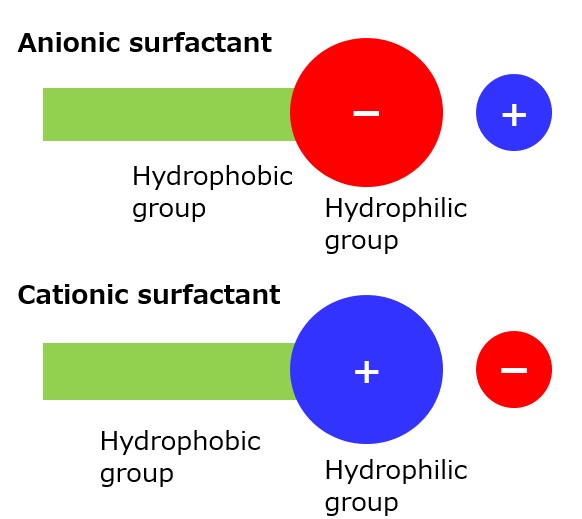
Fig. Model diagram of anionic and cationic surfactants
Basic Structure of Cationic Surfactants
In view of their form, the structure of cationic surfactants is quite the opposite of that of anionic surfactants. As shown in the figure to left, the hydrophilic groups are ionized into cations when the surfactants are dissolved in water. Therefore, they are sometimes called invert soaps or positive soaps.
Then, what kind of hydrophilic groups should be introduced in order to create a positive charge in this way?
Hydrophilic Groups of Cationic Surfactants
A sodium ion (Na+) and a potassium ion (K+) are certainly hydrophilic groups with positive charges, but there is no way to combine them with hydrophobic groups. However, among the same inorganic cations, it seems possible to find a way of combining an ammonium ion (NH4+) with hydrophobic groups. For instance, it might be possible to substitute a hydrogen atom of ammonium chloride (NH4+Cl-) with a hydrophobic group such as an alkyl group.
In fact, a hydrogen atom can be substituted with an alkyl group, and furthermore, not only one, but also two, three or four hydrogen atoms can be substituted freely. That is, four kinds of substituted ammonium chlorides, each having a specific name, can be prepared, as shown in the table below.
Ammonium chloride with alkyl groups
| Number of alkyl group | Kind of ammonium salt | Alternative name, taking into account the neutralization of amine with hydrochloric acid |
|---|---|---|
| 0 | 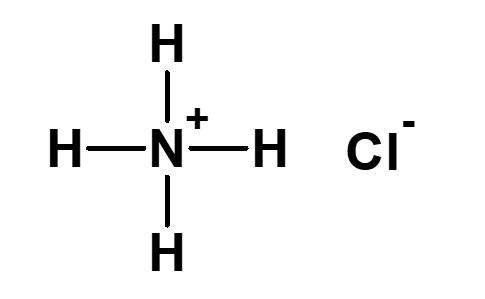 Ammonium chloride |
NH3・HCl Hydrochloric acid salt of ammonia |
| 1 | 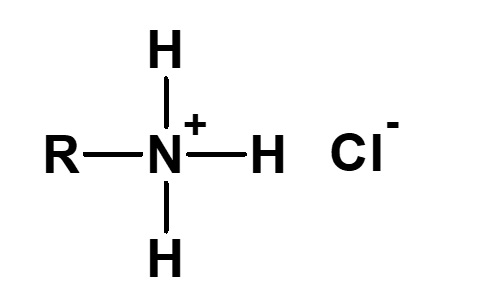 Monoalkylammonium chloride |
R-NH2・HCl Hydrochloric acid salt of primary amine |
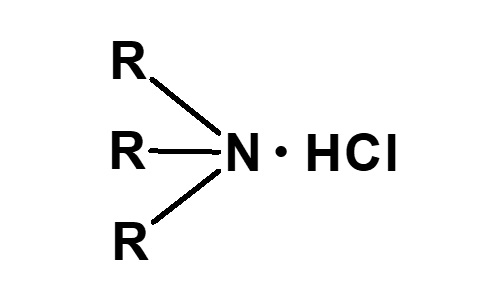
| 2 | 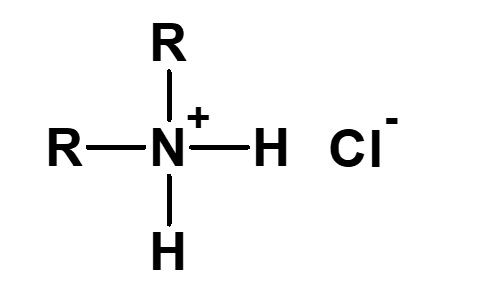 Dialkylammonium chloride |
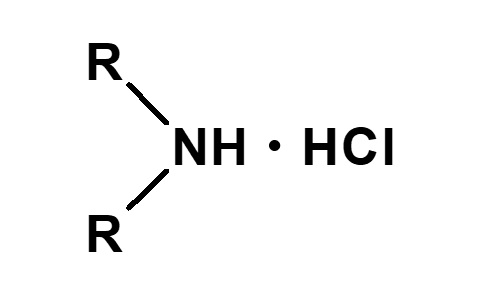 Hydrochloric acid salt of secondary amine |
| 3 | 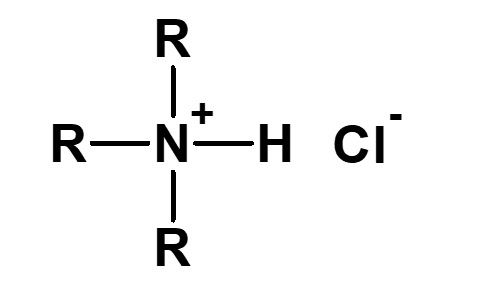 Trialkylammonium chloride |
 Hydrochloric acid salt of tertiary amine |
| 4 | 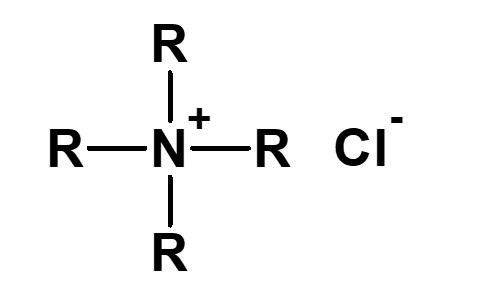 Tetraalkylammonium chloride (quaternary ammonium chloride) |
This special compound cannot be split into amine and hydrochloric acid. Its nature is also very different from that of others. The following schemes are conceivable if it is forced to split in two: a) R4N+OH- + HCl → R4N+Cl- + H2O b)R3N + RCl → R4N+Cl- |
Synthesis of Cationic Surfactants by Neutralization of Amines
In actual practice they are not manufactured by substitution of hydrogen atoms of ammonium chloride with alkyl groups. Instead, just as ammonium chloride is prepared by neutralization of ammonia with hydrochloric acid, alkylated ammonium chlorides (hydrochloric acid salts of amines) with up to three alkyl groups are manufactured easily by neutralization with hydrochloric acid of appropriate primary amines, secondary amines or tertiary amines, respectively, as shown below.
Thus, higher alkylamines can be converted easily to cationic surfactants by simple neutralization with acids. Accordingly, not only strong inorganic acids such as hydrochloric acid, but also relatively weak lower fatty acids such as formic acid and acetic acid may yield amine salt type cationic surfactants by neutralizing amines with them.
Examples of salts formed by the neutralization reaction of amine compounds with acids
| Amine compound | Acid | Product | ||
|---|---|---|---|---|
| NH3 | HCl | → | NH3・HCl | NH4+Cl- |
| RNH2 | HCl | → | RNH・HCl | RNH3+Cl- |
| R2NH | HCl | → | R2NH・HCl | R2NH2+Cl- |
| R3N | HCl | → | R3NH・HCl | R3NH+Cl- |
Examples of laurylamine acetate
For example, acetic acid salt of laurylamine is obtained as a result of exothermic neutralization by adding a calculated amount of acetic acid during stirring to laurylamine (C12H25NH2, a white, waxy solid insoluble in water), which has been melted by heating to 60 to 70 °C.
Acetic acid salt of laurylamine thus obtained is an excellent surfactant which is easily soluble in water.
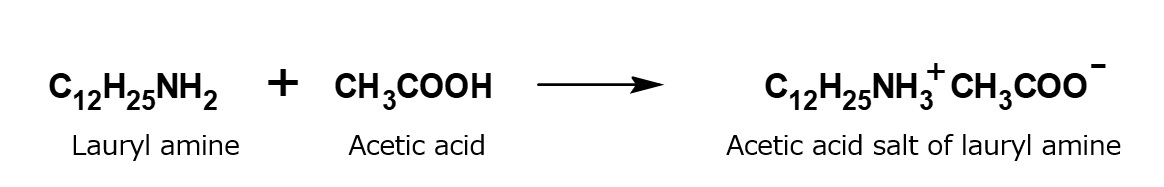
Fig. Formation reaction of laurylamine acetate
Raw Material Amines for Cationic Surfactants
In this connection, special attention should be paid to the fact that neutralization of higher alkylamines with acids such as higher fatty acids, which are difficult to dissolve in water, results in formation of salts of amines that are insoluble in water.
Then, what kinds of amines can be used as raw materials for the cationic surfactants mentioned above ? To make you familiar with these amines, The table below shows not only higher alkylamines that are convertible into surfactants directly but also lower alkylamines, which are important as intermediates.
Examples of raw material amines for cationic surfactants 1 (alkyl amines, ethanolamines)
| Type | Primary amine | Secondary amine | Tertiary amine |
|---|---|---|---|
| Alkylamines | CH3ーNH2 (Monomethylamine) |
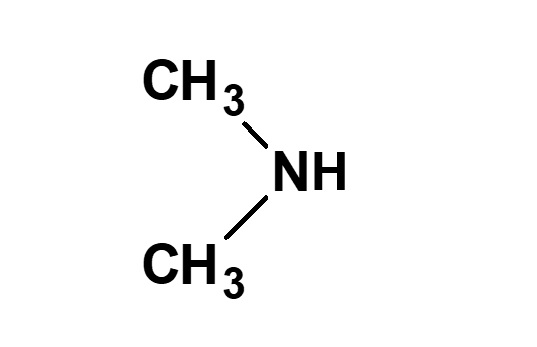 (Dimethylamine) |
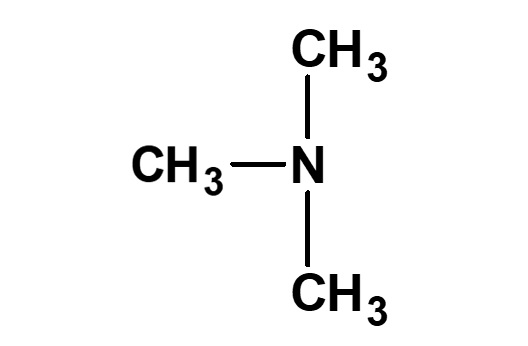 (Trimethylamine) |
| C12H25ーNH2 (Laurylamine) |
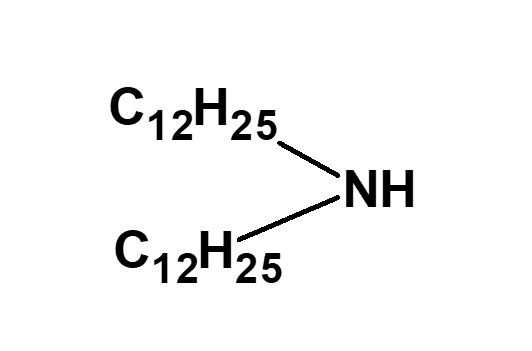 (Dilaurylamine) |
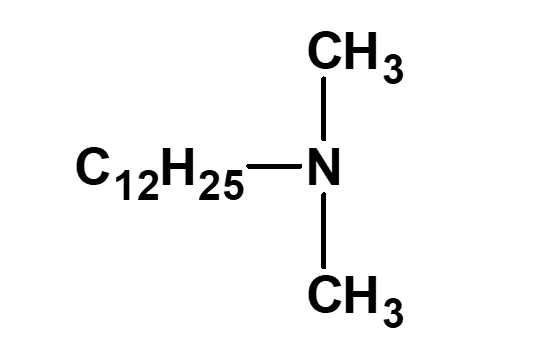 (Lauryldimethylamine) |
|
| C18H37ーNH2 (Stearylamine) |
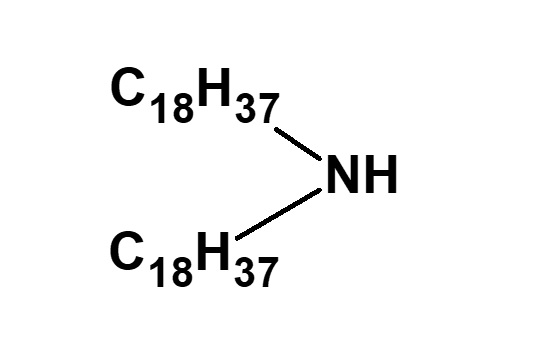 (Distearylamine) |
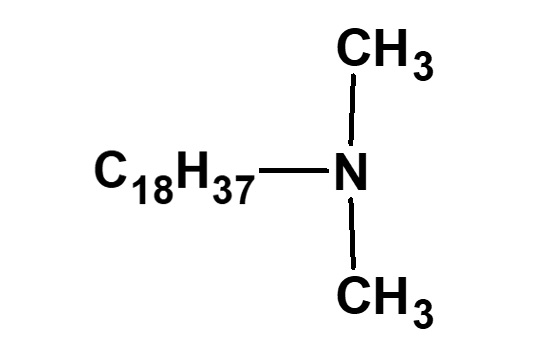 (Stearyldimethylamine) |
|
| Ethanolamines | HOCH2CH2NH2 (Monoethanolamine) |
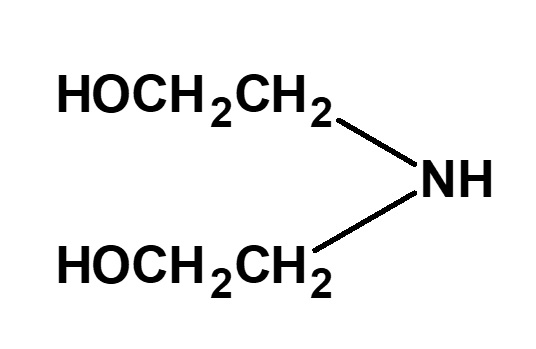 (Diethanolamine) |
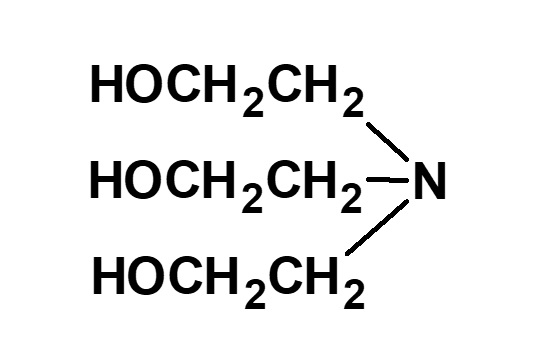 (Triethanolamine) |
Examples of raw material amines for cationic surfactants 2 (polyethylene polyamines and other amines)
| Polyethylene-polyamines | H2NCH2CH2NH2 (Ethylenediamine) H2NCH2CH2NHCH2CH2NH2 (Diethylenetriamine) |
||
|---|---|---|---|
| Other amines | 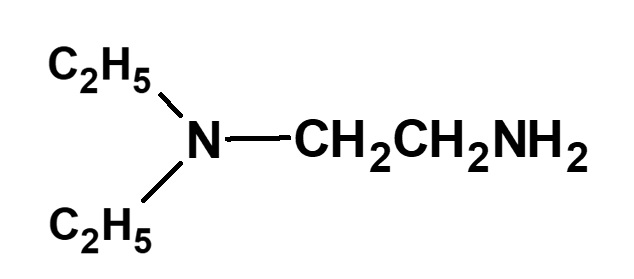 (N,N-Diethylethylenediamine) H2NCH2CH2NHCH2CH2OH (Aminoethanolamine) |
|
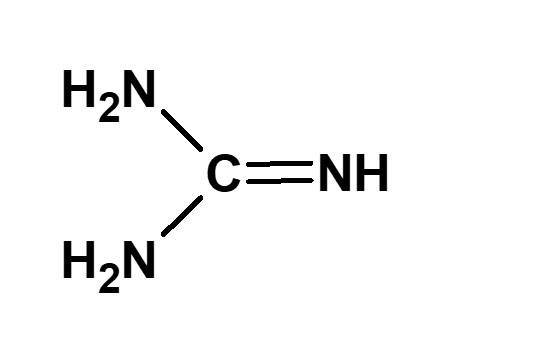 (Guanidine) H2N-NH2 (Hydrazine) |
Synthesis of quaternary ammonium chlorides with alkylating agents
Incidentally, you may note that mono-, di- or trialkylammonium chloride (hydrochloric acid salt of primary, secondary or tertiary alkylamine) can certainly be prepared by the process mentioned above, but tetraalkylammonium chlorides (quaternary ammonium chlorides) cannot be prepared in the same way In other words, it is impossible to prepare alkylammonium chlorides with four alkyl groups that are linked to the central nitrogen atom by neutralizing of any kind of amine with hydrochloric acid.
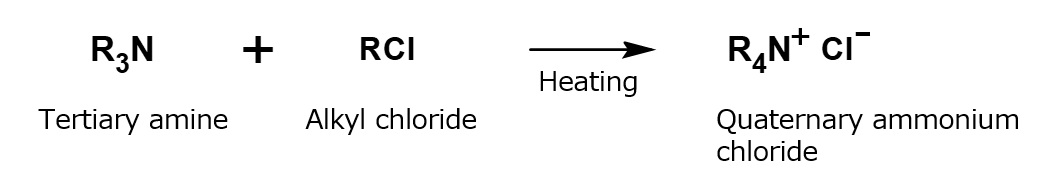
Fig. Reaction of tertiary amine with alkyl chloride
(Quaternary ammonium chloride formation reaction)
For the preparation of quaternary ammonium chloride, it is necessary to have tertiary amine react with alkyl chloride.
The reaction does not take place as easily as in the case of neutralization, but it should proceed fairly smoothly as the reaction itself is exothermic.
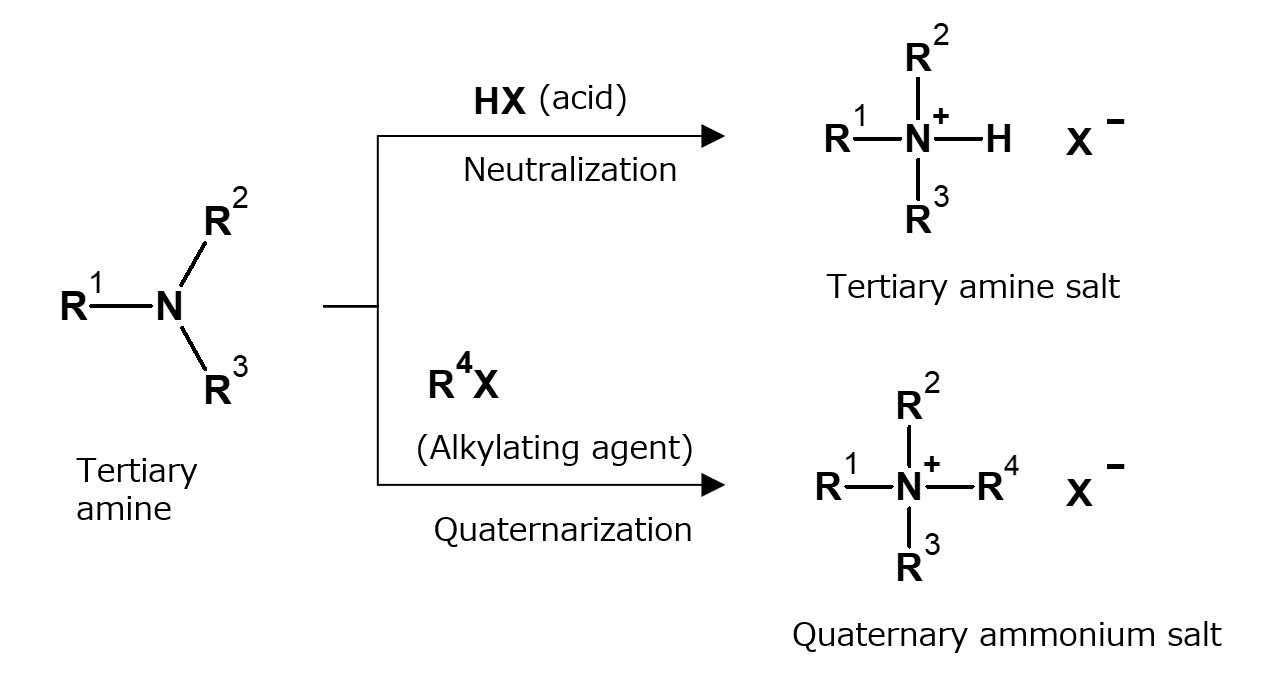
Fig. Formation reaction of amine and ammonium salts by acids and alkylating agents
In general, quaternization of tertiary higher alkylamines with alkylating agents is necessary for the manufacture of quaternary ammonium salt type surfactants.
Alkylating agents are compounds such as methyl chloride (CH3Cl) and dimethyl sulfate [ (CH3O)2SO2) ] that have the property of passing their alkyl groups easily to other compounds.
Therefore, it can be concluded that tertiary amines yield amine salt type cationic surfactants by neutralization with acids, and yield, quaternary ammonium salt type cationic surfactants by reaction with alkylating agents (quaternarization).
The table bellow shows typical examples of alkylating agents which are used frequently to manufacture quaternary ammonium salt type cationic surfactants. These alkylating agents yield quaternary ammonium salts through the following reaction examples.
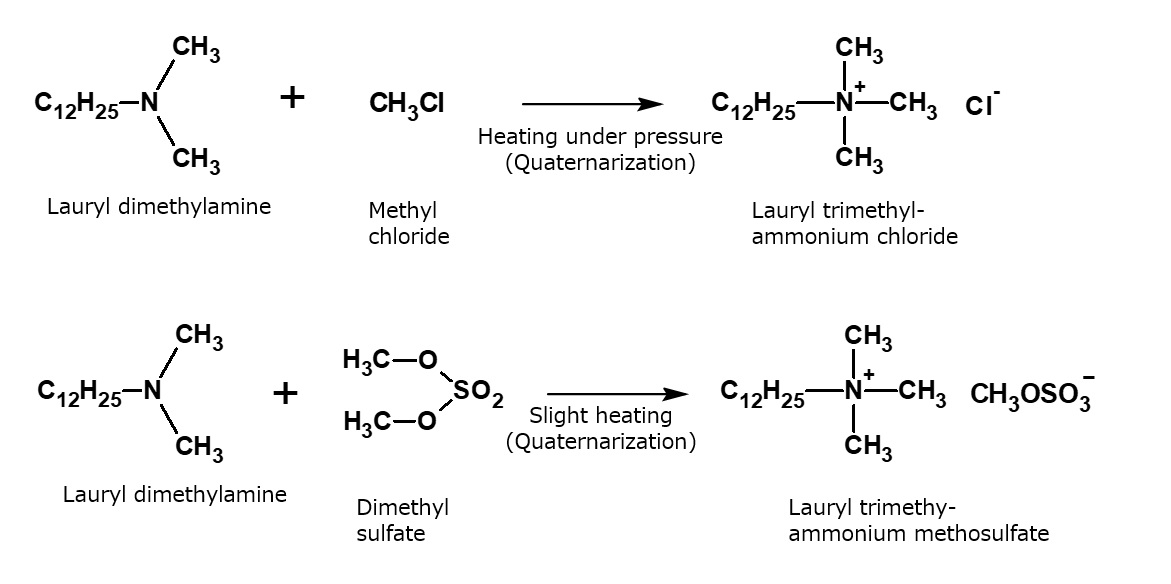
Fig. Formation reaction of quaternary ammonium salts by alkylating agents
Typical alkylating agents used to make quaternary ammonium salts
| Alkylating agent | Type of quaternary ammonium salt |
|---|---|
| CH3Cl Methyl chloride |
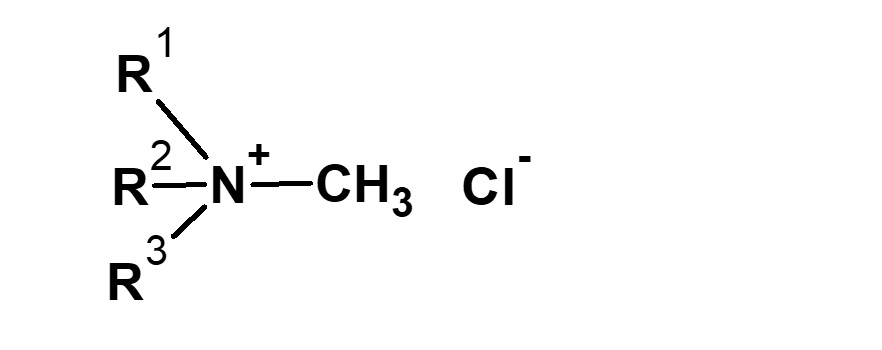 |
| CH3Br Methyl bromide |
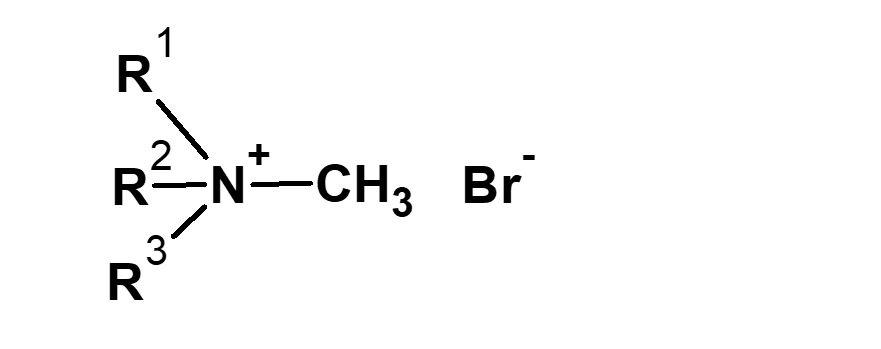 |
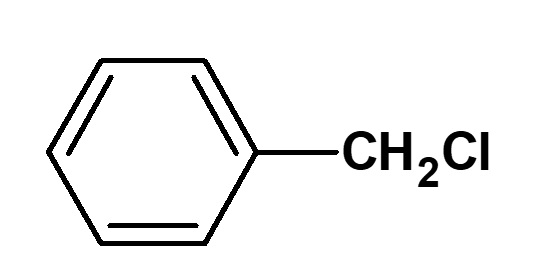 Benzyl chloride |
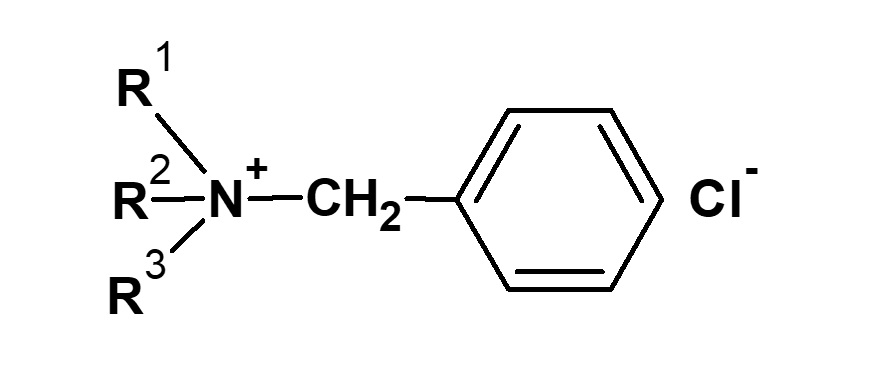 |
| RCl Higher alkyl chloride |
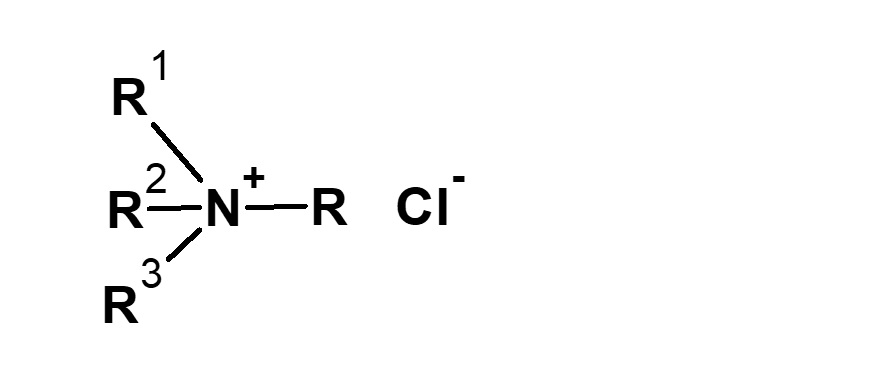 |
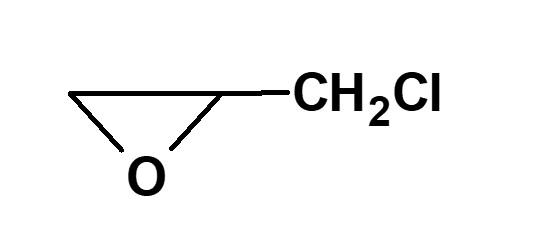 Epichlorohydrin |
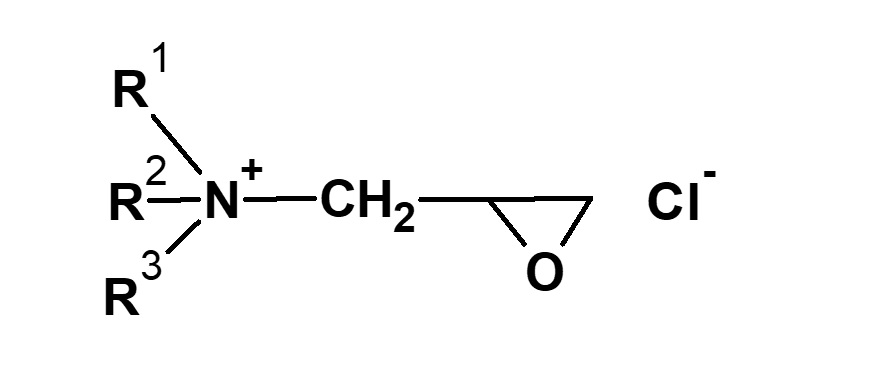 |
| Alkylating agent | Type of quaternary ammonium salt |
|---|---|
| (CH3O)2SO2 Dimethyl sulfate |
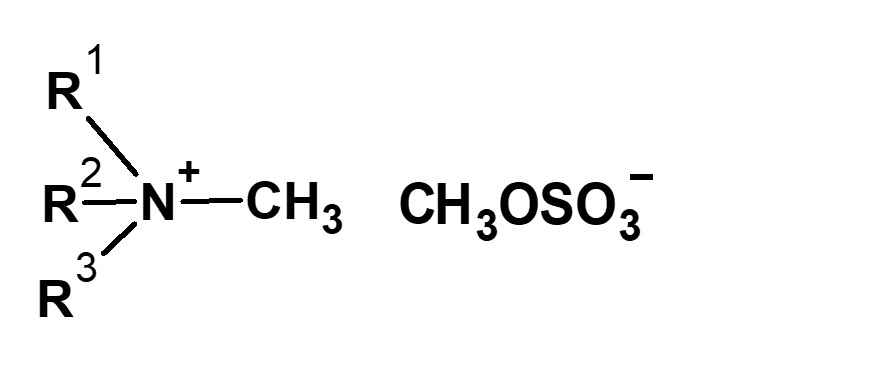 |
| (C2H5O)2SO2 Diethyl sulfate |
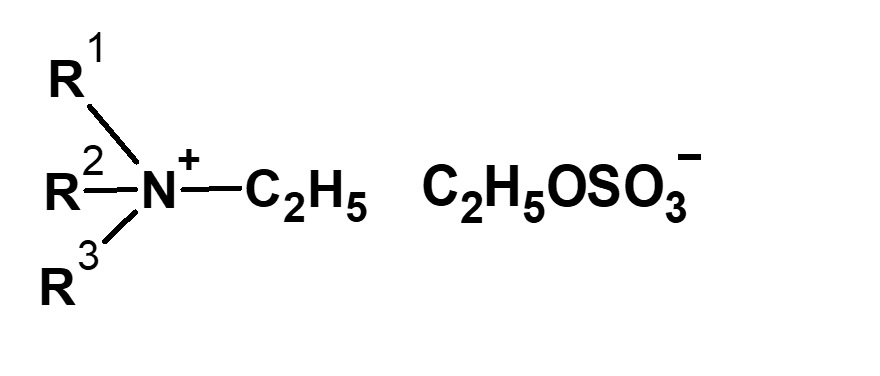 |
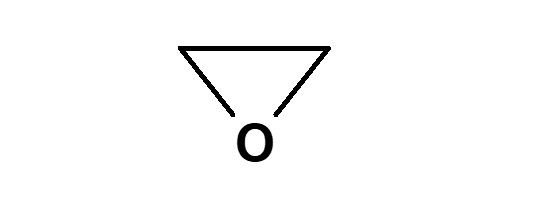 Ethylene oxide |
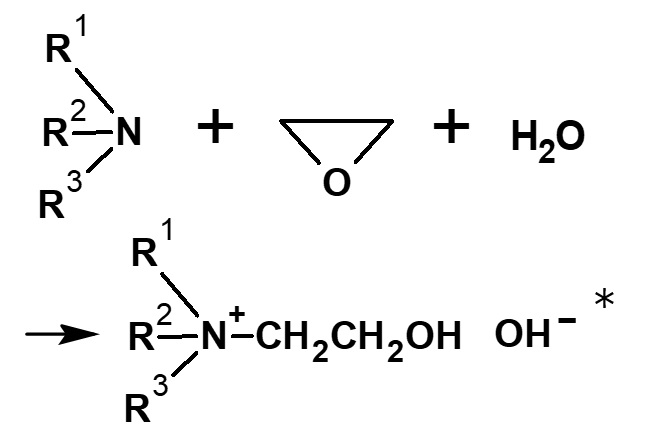 |
| (CH3O)2CO Dimethyl carbonate |
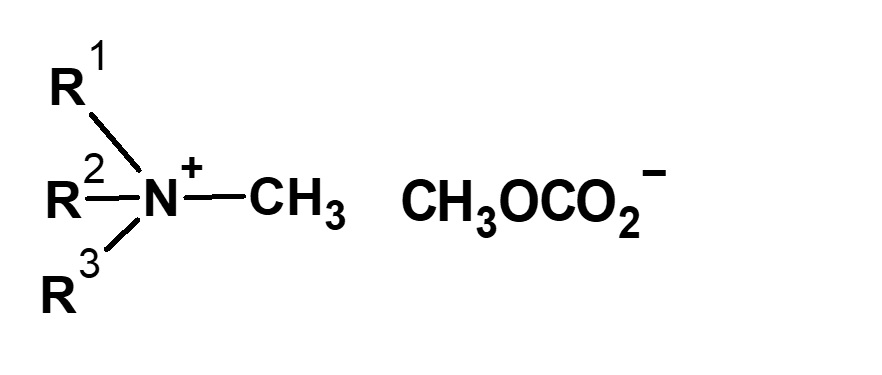 |
Amine Salt Type Cationic Surfactants
In this page, salts of primary, secondary and tertiary amines are called collectively amine salt type cationic surfactants because there are no sharp distinctions among them, as a result of their similar properties. Furthermore, many commercial products contain primary and secondary amines together.
Since products of this type are classified into surfactants, it is no wonder that each of their hydrophobic groups consists predominantly of alkyl groups with carbon numbers from 12 to 18. However, curiously enough, amine salt type cationic surfactants are rarely used for applications that would require surface activity, in the basic sense of the term. Examples will be introduced in the following pages.
Amine Salt Type Cationic Surfactants Derived from Higher Alkylamines
Synthetic method for aliphatic higher amines
Higher alkylamines are synthesized by hydrogenating fatty nitriles, which are prepared by heating fatty acids or esters with ammonia.
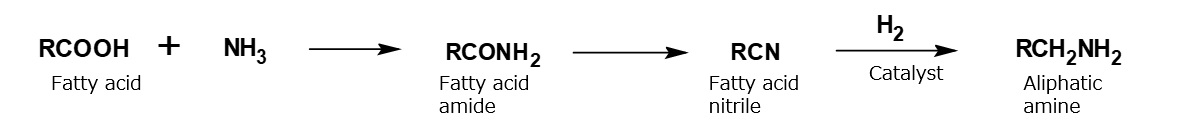
Fig. Synthetic route of aliphatic amines
Coconut amine containing mainly laurylamine (C12), and hydrogenated tallow amine containing mainly stearylamine (C18), are manufactured commercially by this process from coconut oil and tallow, respectively Low-priced amines such as rosin amine produced from rosin acid are also available.
As previously discussed, these amines are converted to surfactants easily by neutralization with acids such as hydrochloric acid and acetic acid. Relatively speaking, however, only a small amount of salts of higher alkylamines is consumed commercially.
Higher alkylamine ethylene oxide adducts
Synthetic method for higher alkylamine ethylene oxide adducts
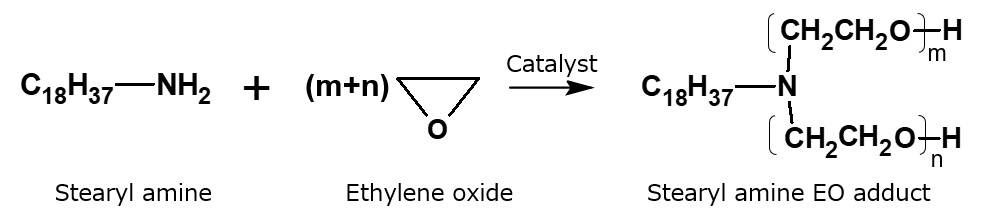
Fig. Ethylene oxide addition reaction to higher alkyl amines
A surfactant having intermediate nature, between a cationic surfactant and a nonionic surfactant, is obtained by reacting a higher alkylamine with ethylene oxide.
Water solubility of higher alkylamine ethylene oxide adducts
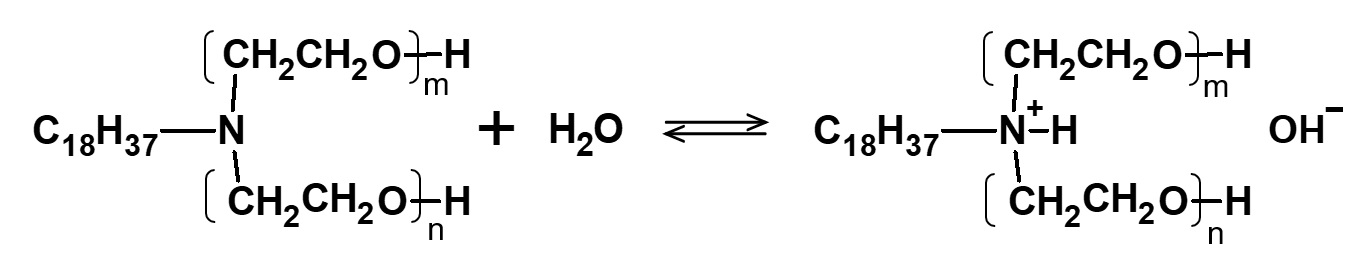
Fig. Cationization of stearylamine EO adduct in water
This type of product is highly soluble in water, whether neutralized with acid or not.
Examples of applications of higher alkylamine ethylene oxide adducts
The effect of this structure's nonion-cation combination is directly apparent in its properties, and it is used relatively widely for special applications, for example, as a dyeing auxiliary.
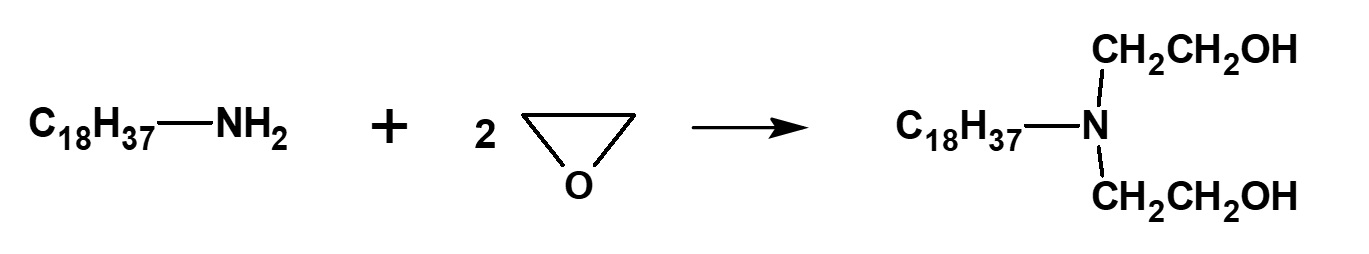
Fig. Formation reaction of dihydroxystearylamine
This kind of surfactant with exactly 2 moles of ethylene oxide can be manufactured by addition of EO to higher alkylamines without a catalyst. Dihydroxyethylstearylamine, a surfactant manufactured by this process, is famous as a polymer-alloy type antistatic agent for plastics.
Amine Salt Type Cationic Surfactants Derived from Lower Alkylamines
Higher alkylamines are fairly expensive, so there is a demand to manufacture cationic surfactants from raw materials that are cheaper. Whatever the approach, there may be no appropriate way to achieve this other than to use something like fatty acid, which is a cheap source of hydrophobic groups. Then, it will be of great practical value to try to synthesize cationic surfactants by reacting fatty acids such as oleic acid and stearic acid with lower alkylamines. In practice, the reaction proceeds very smoothly, and furthermore, it easily yields products with a variety of structures.
In many cases, this series of products is not only inexpensive, but also excellent in performance. Cationic surfactants used as textile softeners generally belong to this series.
Fattyester-amine salt type cationic surfactants
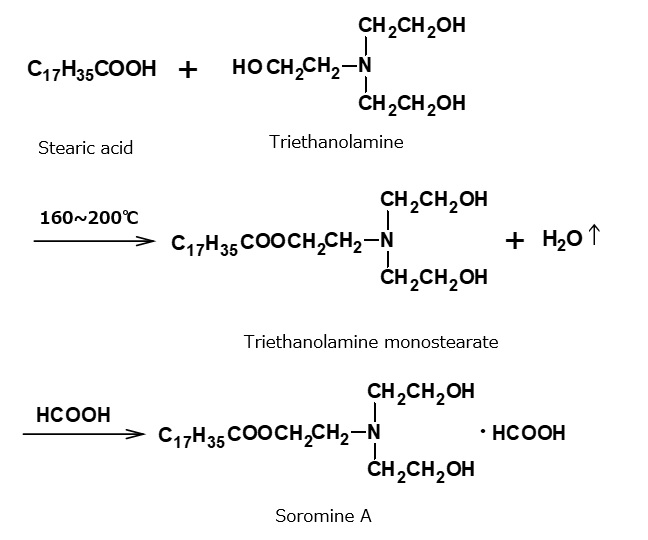
The cationic textile softener Soromine A, developed by the German company formerly known as I.G., belongs to the category fattyester-amine salt type cationic surfactants. Surfactants of this type are also called Soromine A type cationic surfactants.
They are cationic surfactants manufactured by condensation between stearic acid and triethanolamine during heating in order to produce a tertiary amine of fattyester type, and by subsequent neutralization with formic acid.
Soromine A was once so famous that, when many companies became interested in cationic softeners after World War II, manufacture of equivalent products was undertaken. Since then, various improvements have been made, and very complex products in this category are now on the market.
Products in this category in particular are among the less expensive cationic surfactants because they can be manufactured easily with cheap raw materials. They also have high performance, and are still used commercially today. Their disadvantage is the easy hydrolysis of the ester linkage that combines the hydrophobic group with the hydrophilic group. Hydrolysis is prevented to some extent when strong acid is used for neutralization of the amine.
Fattyamide-amine salt type cationic surfactants
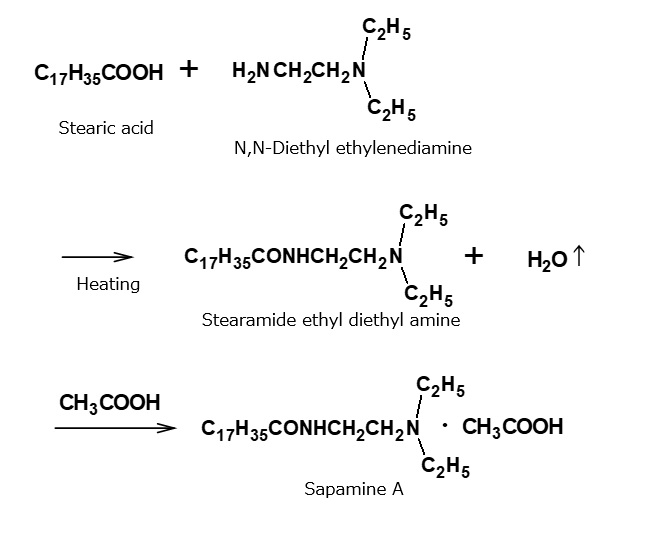
Sapamine A, developed by CIBA of Switzerland, belongs to fattyamide-amine category of salt type cationic surfactants. Surfactants of this type are also called Sapamine A type cationic surfactants.
This cationic surfactant is manufactured by condensation between N,N- diethylethylenediamine and stearic acid during heating, and subsequent neutralization with acetic acid.
Products of this type are also used as softeners, and they differ from Soromine A type products in that they possess a strong resistance to hydrolysis due to the amide linkage between the hydrophobic and hydrophilic groups. On the other hand, however, they are more expensive than Soromine A type products due to the fact that the amine source is not a commodity but a specialty chemical.
Urea-amine salt type cationic surfactants
Ahcovel A is a typical product among the Ahcovel series of cationic softeners, first developed by Arnold Hoffman Co. of the USA in the 1940s.
Ahcovel A is manufactured by a series of complicated reactions. An intermediate obtained by condensation between stearic acid and aminoethyl- ethanolamine during heating is reacted with urea, and the resultant amine is neutralized by acetic acid.
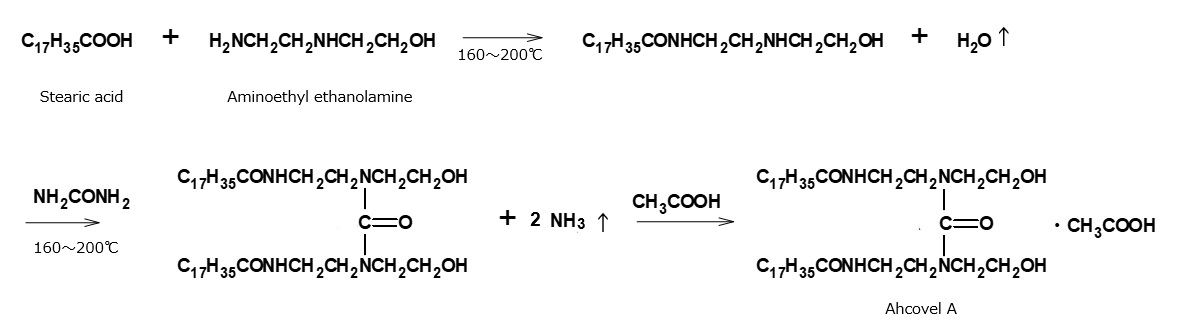
Fig. Synthesis route of urea condensed amine salt type cationic surfactant (Ahcobel A)
Close examination of the structural formula of Ahcovel A reveals that it does not contain any amino group. If so, how is neutralization with acetic acid possible? As the above formula appears in a patent owned by that company, this is the formula that has generally been in use.
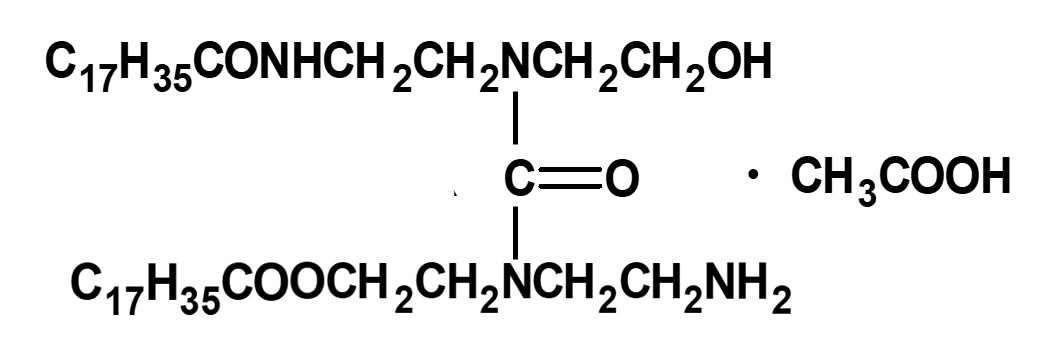
Fig. Principal Components of Ahcobel A (Estimated)
As a matter of fact, however, Ahcovel A is not the simple compound shown above but a mixture of products resulting from complex reactions.
According to the results of research conducted by Dr. Fujimoto et al., it seems that the main component is a compound with a structure close to the left.
Urea condensed amine salt type cationic surfactant applications
This Ahcovel series of products shows excellent performance as textile softeners, and many companies supply equivalents. Other amines such as diethylenetriamine are also used in some cases. Cationic surfactants sold as textile softeners of this kind are supplied generally in the form of a white paste of aqueous emulsion with a concentration of 20% to 40%. When warm water is added during stirring, a dilute emulsion with fine and uniform particles is easily obtained.
Imidazoline type cationic surfactants
When amines such as aminoethylethanolamine and polyethylenepolyamine are reacted with fatty acids at 160 to 180 °C, amines in the form of amide are obtained, as discussed above. When they are reacted at higher temperatures between 200 and 250 °C, amines of a new type called imidazoline derivatives are obtained. Imidazoline derived from oleic acid and aminoethylethanolamine is shown in the following formula, but it is actually not as simple as the compound shown in this formula. Rather, it is a mixture of products resulting from cleavage of an imidazoline ring.
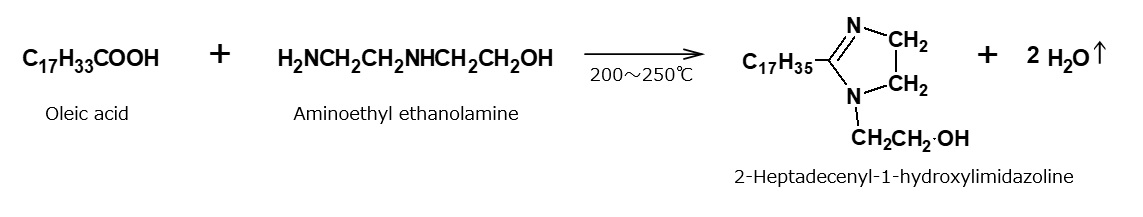
Fig. Example of formation reaction of imidazoline derivatives
Imidazoline type cationic surfactants derived from oleic acid can be used as emulsion breakers. They are also used as intermediates for further reactions to manufacture quaternary ammonium salt type cationic surfactants or amphoteric surfactants.
Onyxan HSB (Refined-Onyx Div., Milmaster Onyx Corp.) is a famous textile softener in this category, and its nature is very similar to that of Ahcovel G. Imidazoline type cationic surfactants derived from lauric acid are used as intermediates to manufacture amphoteric surfactants.
Quaternary Ammonium Salt Type Cationic
Surfactants
Quaternary ammonium salt type cationic surfactants are manufactured by means of the reaction between tertiary amines and alkylating agents. Various combinations of the two reactants yield a great variety of products.
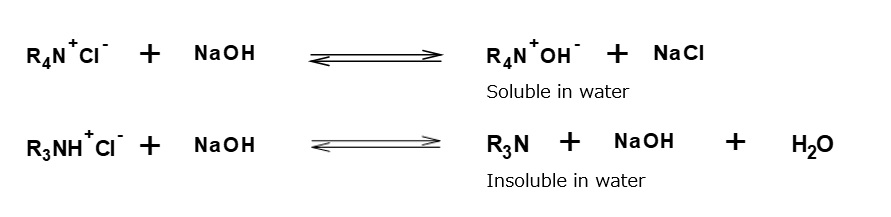
Quaternary ammonium salts are generally no longer decomposed to regenerate the original amines, even if strong alkalis such as sodium hydroxide are added to the aqueous solution.
On the contrary, in the case of salts of amines, original amines that are insoluble in water are regenerated in alkaline solution because acids combined with amines are easily detached with sodium hydroxide. This property of quaternary ammonium salts is very important and should be remembered.
Quaternary Ammonium Salt Type Cationic Surfactants Derived from Higher Alkylamines
Alkyltrimethylammonium salts
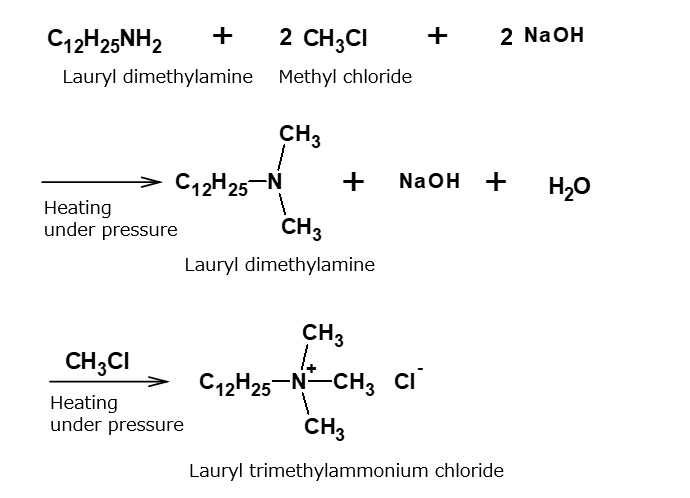
Fig. Synthetic route of lauryl trimethylammonium chloride
When higher alkylamines are reacted with methyl chloride under pressure in the presence of sodium hydroxide, tertiary amines are produced, and then converted into quaternary ammonium salts.
Products of this series are typical quaternary ammonium salts that dissolve in water transparently and have fairly good surface activity. They are used, for instance, as an additive for viscose coagulation baths.
Alkylbenzyldimethylammonium salts
These quaternary ammonium salts, which have particularly strong germicidal properties, are produced by alkylating alkyldimethylamine (tertiary amine) with benzyl chloride. Alkyl groups in the vicinity of lauryl (C12) are preferred.
These compounds are also called benzalkonium chlorides, and are produced in fairly large quantities as germicides. They dissolve in water transparently and generate large amounts of foam. They are used widely in hospitals and similar facilities as their germicidal effect is about 500 times stronger than that of phenol. Furthermore, these compounds have no smell and will not irritate the skin.
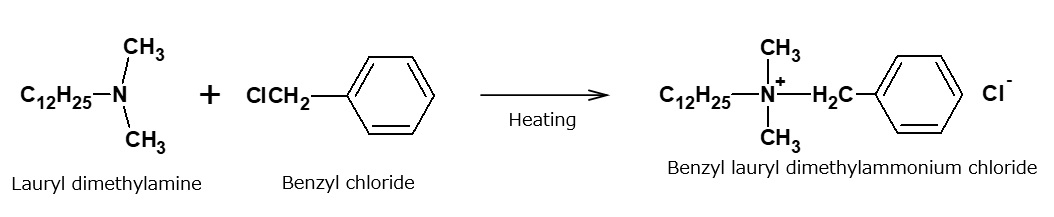
Fig. Synthesis route of benzylauryl dimethyl ammonium chloride (benzalkonium chloride)
Quaternary ammonium acylate
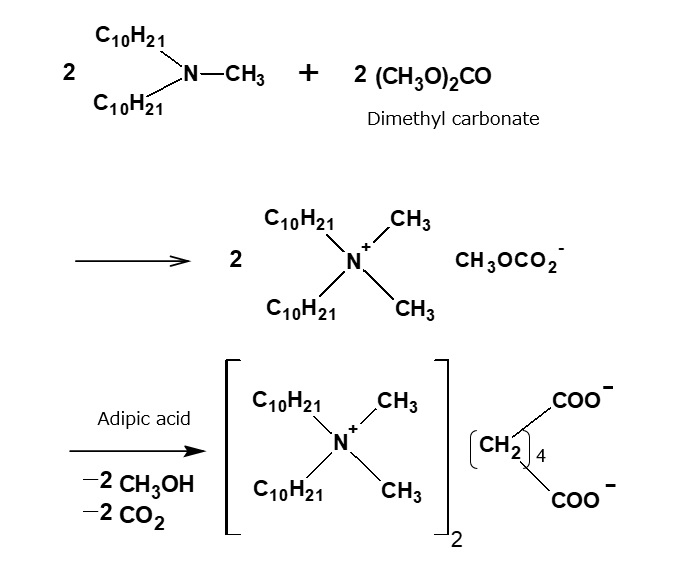
Fig. Adipate of quaternary ammonium
As halogen ions corrode metal, there is a demand for quaternary ammonium salts that contain no halogen ions. These compounds are now being developed. As the following reaction formula shows, when the quaternary ammonium salt synthesized through the reaction of a tertiary amine and dimethyl carbonate is treated with an organic acid, a quaternary ammonium acylate is obtained.
When adipic acid is used as the organic acid, a quaternary ammonium acylate is obtained. This compound has the property of preventing corrosion of metal (rust preventive effect).
Quaternary Ammonium Salt Type Cationic Surfactants
Derived from Lower Alkylamines
It is possible to manufacture inexpensive quaternary ammonuim salts starting with lower alkylamines and cheap hydrophobic groups. As this result in a wider choice of structures and properties, there has been a lot of development and diversification of this type of surfactant.
Fattyamide-ammonium salt type quaternary ammonium salts
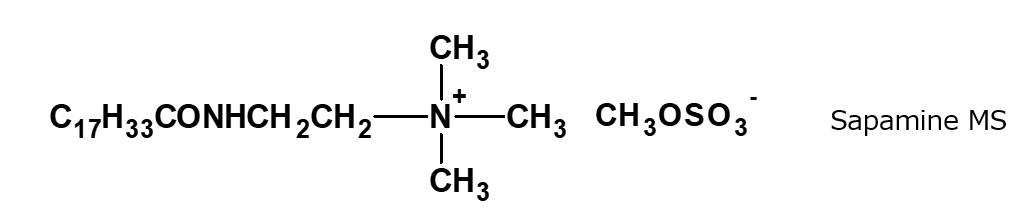
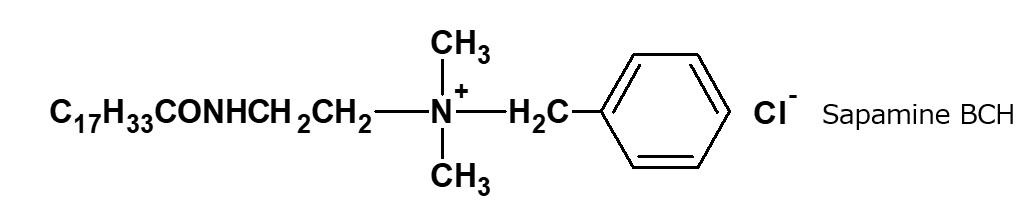
Fig. Example of fatty acid amide type quaternary ammonium salt
Tertiary amines are produced by condensation of N,N-diethylethylenediamine with fatty acids, and quaternary ammonium salt type surfactants are obtained by alkylating the resultant tertiary amines with various alkylating agents. For example, the following surfactants developed by CIBA of Switzerland belong to this category.
Their applications as fixing agents for direct dyes and textile softeners are well known. However, they are no longer used as fixing agents due to recent progress in the development of non-surfactant type products with superior performance.
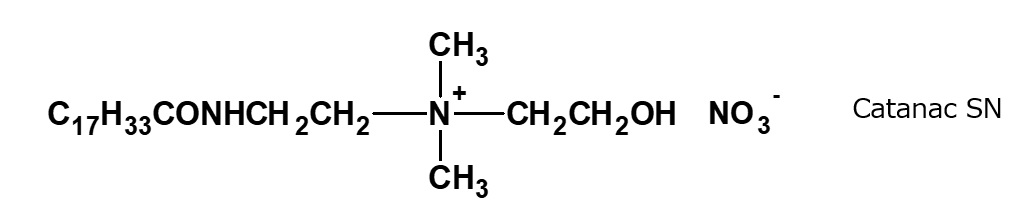
Fig. Structural Formula of Catanac SN
Catanac SN (American Cyanamid Co.), which has a similar structure to that of Sapamines was once well known as an antistatic agent for synthetic resins.
This product can be synthesized easily by the reaction of ethylene oxide with tertiary amine nitrate, but it is no longer in use.
Pyridinium salts
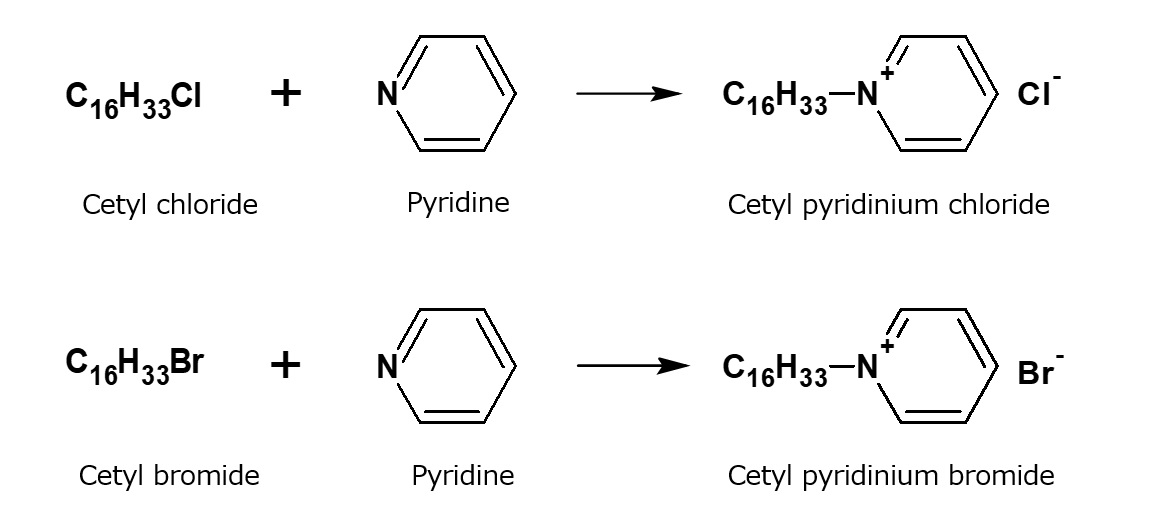
Fig. Example of reaction to form alkylpyridinium salts
Pyridine is a tertiary amine with the unique structure.
Alkylation of pyridine with higher alkyl chloride or bromide yields alkylpyridinium salts that are equivalent to quaternary ammonium salts.
These products can be used as dyeing auxiliaries or as germicides. Especially, cetylpyridinium chloride is used as a germicide in toothpaste.
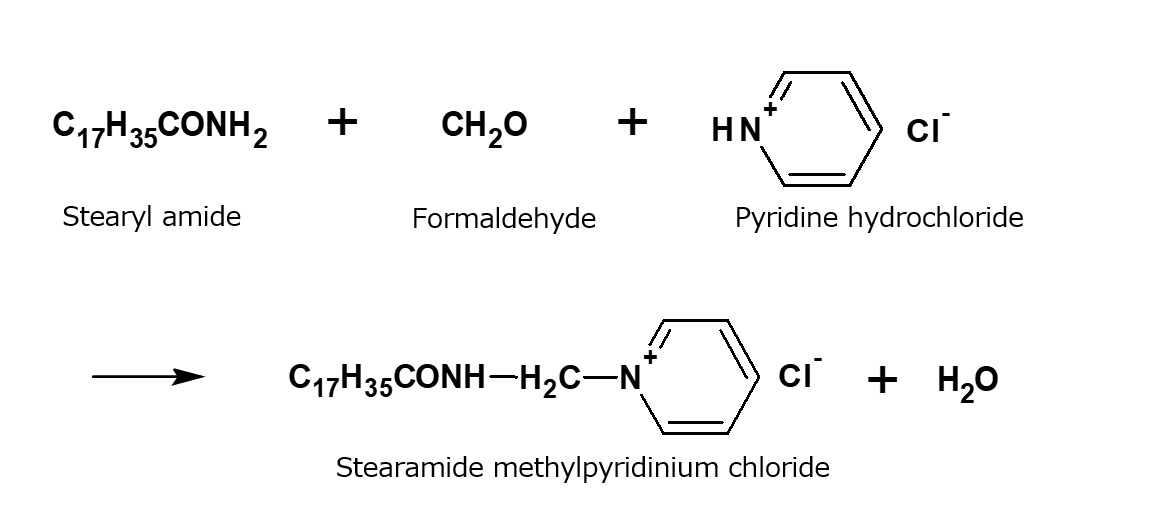
Fig. Example of reaction to form alkylpyridinium salts
The largest market for pyridinium salts is the use for water-repellent finishing of textiles. Special pyridinium salts in the form of stearic acid amide and pyridine hydrochloride linked with formaldehyde were used.
They can be dispersed easily in water. When textiles treated with these products are heated, a durable water-repellent effect is imparted, as a result of the amide portion of the molecules being left on the textiles after thermal decomposition of the pyridinium salts. These products can be said to form a particular group of their own among cationic surfactants. At present, however, they are considered favorable for use due to the terrible smell of pyridine.
Summary of Cationic Surfactants
Cationic surfactants are divided broadly into two categories, amine salt type and quaternary ammonium salt type. These two types have different properties and manufacturing processes. It is very important to understand the differences between the two types, as summarized in the table bellow.
Difference between amine salt type and queternary ammonium salt type cationic surfactants
| Process and properties | Amine salt type | Quaternary ammonium salt type |
|---|---|---|
| Manufacturing process | Neutralization of amines with acids | Quatenarization of tertiary amines with alkylating agents |
| Water solubility | Relatively low | Relatively high |
| Stability of aq solution | Stable only in acid solutions; Unstable in neutral to alkaline solution |
Generally stable in acid to alkaline solution |
| Germicidal effect | Generally none | Generally high |
On mixing cationic and anionic surfactants
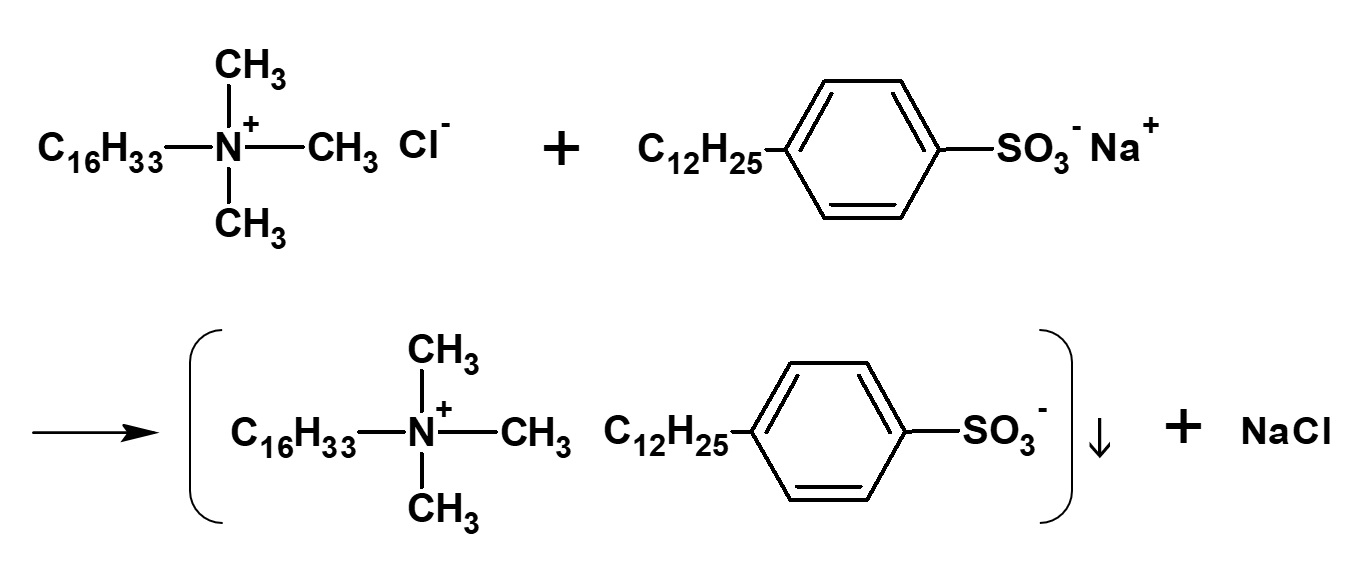
Fig. Examples of products when mixing cationic and anionic surfactants
Cationic surfactants have ionic activity of a completely opposite nature to that of anionic surfactants, and therefore, these two kinds of surfactants cannot be used together. That is to say, if aqueous solutions of these surfactants are mixed together, precipitation will occur and they will cease to function.
The above fact is one of the major causes for failure often experienced in the practical application of surfactants. For example, soaking a fabric that has been washed with anionic detergent in a cationic finishing bath, without rinsing it first, is something that should be avoided.
Properties of cationic surfactant solutions
Aqueous cationic surfactant solutions are generally acidic in contrast to those of anionic surfactants, which are generally neutral or alkaline. That is, roughly speaking, cationic surfactants ionize into weaker base and stronger acid, and anionic surfactants ionize into weaker acid and stronger base. The table bellow explains the matter to enable better understanding by comparing equivalent inorganic compounds.
Nature of aqueous solutions of anionic and cationic surfactants
| Surfactant | Example | Nature of aq solution | Equivalent simple compound |
|---|---|---|---|
| Anionic surfactant |
Soap
RCOO-Na+ |
Weakly alkaline | Na2CO3 |
| Salt of higher alcohol sulfate ROSO3-Na+ |
Neutral | Na2SO4 | |
| Cationic surfactant | Quaternary ammonium salt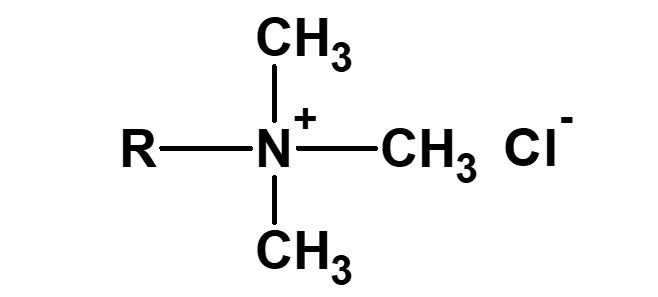 |
Neutral - weakly acidic | NaCl or (CH3)4N+Cl- |
|
Salt of higher alkylamine
RNH3+Cl- |
Weakly acidic | NH4+Cl- |
The table bellow shows the usual classification of cationic surfactants according to the combination of hydrophobic and hydrophilic raw materials.
Classification of cationic surfactants
| Hydrophobic raw material | |||||
|---|---|---|---|---|---|
| Higher alkylamine Primary: RNH2 Tertiary: RN(CH3)2 |
Higher alkyl halide RCl, RBr |
Higher fatty acid RCOOH |
Higher fatty acid amide RCONH2 |
||
| Hydrophilic raw material |
Inorganic acid organic acids |
Salt of higher alkylamine | |||
| Alkylating agents | Quaternary ammonium salts such as benzalkonium chloride |
||||
| Ethanolamines | * | Soromine A type | |||
| Asymmetric diamines such as N,N-diethylethylenediamine |
* | Sapamine type -Sapamine A, CH(tertiary) -Sapamine MS, BCH(quaternary) Catanac SN |
|||
| Aminoethylethanolamines, Polyethylenepolyamines |
* | Ahcovel type Imidazoline type -Onyxan HSB |
|||
| Pyridine | Alkyl pyridinium salt | Zelan and Velan type water-repellent agents | |||
| Ethylene oxide | 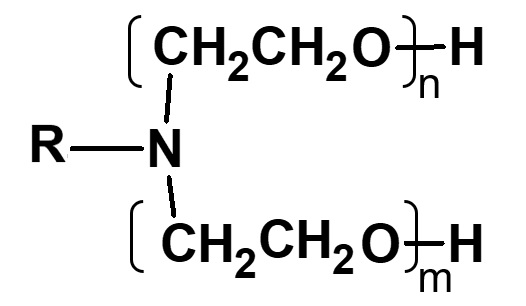 |
* | |||
In this page, discussion of cationic surfactants is limited to only nitrogen compounds. In fact, all commercial products of this type are nitrogen compounds. From the scientific point of view, however, some sulfur compounds, such as sulfonium salts, and some phosphorus compounds, such as phosphonium salts, are also regarded as cationic surfactants.
Related Products & Topics
Related products

- 帯電防止剤
Cationic surfactant type antimicrobial agent "CATION DMS-75E"
Even at low concentrations, it inhibits the growth of Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and black mold, etc.

- 帯電防止剤
Low metal corrosive cationic surfactant type antimicrobial agent "OSMORIN DA-50"
A halogen-free antimicrobial agent that does not generate dioxin when incinerated and has low metal corrosiveness.

- Nonion surfactant
Polyoxyalkylene alkylamine-based surfactant "PUREMEEL EP-300S"
| Links to Sanyo Chemical's corporate site |
|---|
Imidazoline-Type Base Material for Household Fabric SoftenersCationic Surfactant Type Antibacterial Agent for Industrial UseBase Materials for Hair Rinse/Hair Treatment/Hair ConditionerECONOL TM-22 Germicides (Pharmaceutical Use) |
Topics




References
T.Fujimonto, Introduction to Surfactants, Sanyo Chemical Industries, ltd.
This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.