Introduction to Amphoteric Surfactant
What is a surfactant?
First, an interface is a boundary surface that exists between two substances with different properties, and interfaces exist between liquids and solids, liquids and liquids, and liquids and gases.
Surfactants enhance performance by performing functions such as washing, emulsifying, dispersing, wetting, and penetrating at this interface.
Interface = a boundary surface that exists between two substances with different properties
Liquid and solid: cup and coffee, machine and lubricant
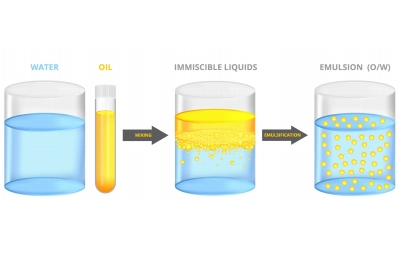
Liquid and liquid: water and oil
Liquid and gas: seawater and air, soap bubbles
Examples of roles of surfactants
Cleaning ・・・ Removing dirt
Emulsification ・・・ Dispersion ・・ Making unmixable things easier to mix
Wetting / Penetration ・・・ Makes wetting and soaking easier
Basic structure of a surfactant
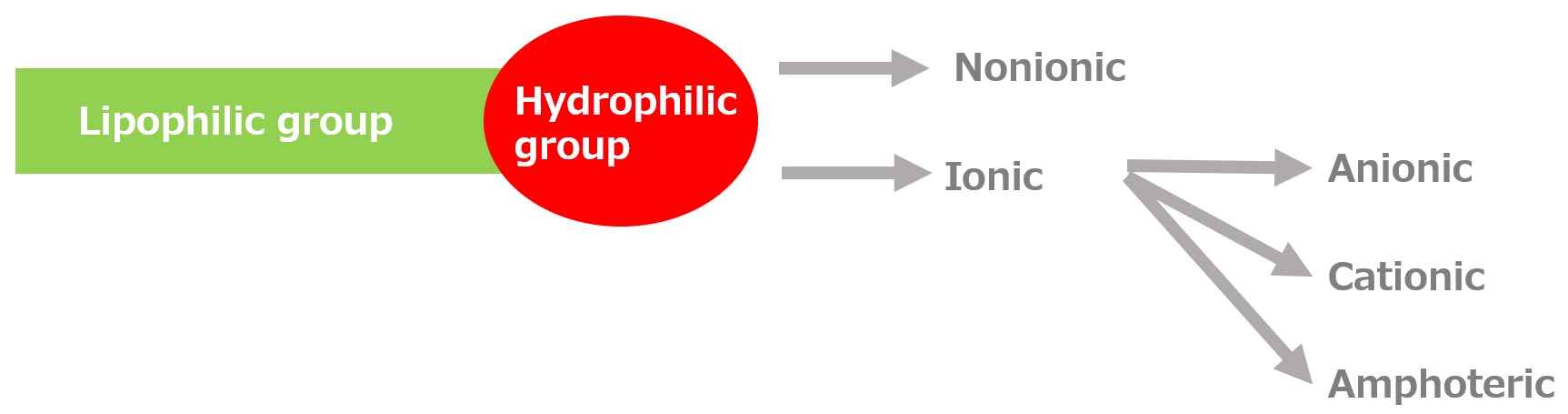
-Surfactants have different structures in their molecules with different properties: lipophilic groups (oil-loving parts) and hydrophilic groups (water-loving parts).
-Surfactants are broadly classified into four types according to the structure of the hydrophilic group: nonionic, anionic, cationic, and amphoteric (having both anionic and cationic groups).
| Type of surfactant | Feature | Main application | Composition example |
|---|---|---|---|
| Nonionic surfactant |
-Hydrophilic and hydrophobic balance can be easily adjusted -Excellent emulsification and solubilization -Low lather -Susceptible to temperature but not to pH |
-Laundry detergent -Emulsifiers and solubilizers -Dispersant -Metalworking oil |
-Polyoxyethylene alkyl ether etc. |
| Anionic surfactant |
-Excellent emulsification and dispersibility -Good lather -Temperature insensitive |
-Laundry Detergent -Shampoo -Body soap |
-Alkyl benzene sulfonate -Alkyl ether sulfates etc. |
| Cationic surfactant |
-Adsorption to fibers -Antistatic effect -Bactericidal |
-Hair rinse -Fabric softener for clothes -Disinfectant |
-Didecyldimethylammonium methyl sulfate |
| Amphoteric surfactant |
-Mild on skin -Excellent solubility in water -Synergistic with other active components |
-Shampoo -Body soap -Kitchen detergent |
-Alkyl di-aminoethyl hydrochloride glycine -Sodium lauryl aminopropionic acid -Dimethylstearyl betaine -Coconut oil fatty acid amidopropyl betaine |
Surfactant functions introduction video
It consists of seven short movies for each function.
0:00 Introduction of surfactants functions
0:16 Part1 Washability
1:00 Part2 Permeability
2:10 Part3 Dispersion
2:55 Part4 Foaming properties
3:25 Part5 Defoaming properties
3:39 Part6 Smoothness
4:20 Part7 Antibacterial properties
What is an amphoteric surfactant?
Strictly defined amphoteric surfactant
Amphoteric surfactants ordinarily are restricted to compounds with an anion-cation combination. In other words, if a surfactant is referred to simply as an amphoteric surfactant, one would be right in assuming it a surfactant with a hydrophobic group to which both an anion and a cation are attached, as shown in (1) of the figure on the right.
Amphoteric surfactants in the broad sense
Broadly speaking, amphoteric surfactants include all hybrid compounds that possess concurrently structures combining any two properties of anionic, cationic or nonionic natures. As shown in the figure on the right,a total of three kinds of such combinations are conceivable.
(1)Combination of anion and cation
(strict definition of amphoteric surfactant)
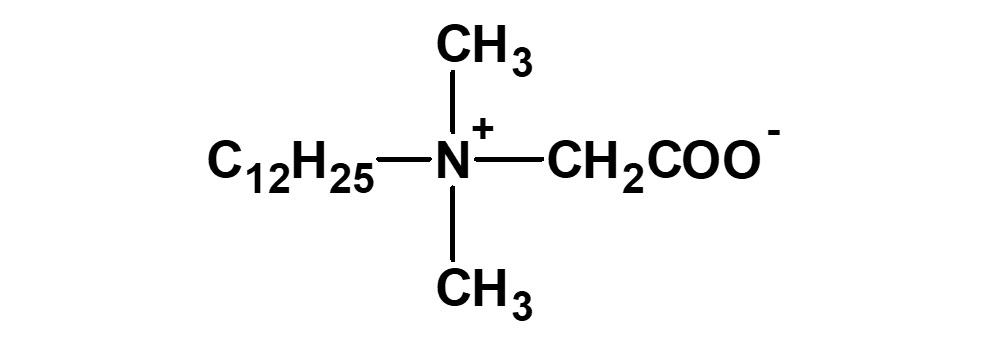
(2)Combination of anion and nonion
![]()
(3)Combination of cation and nonion
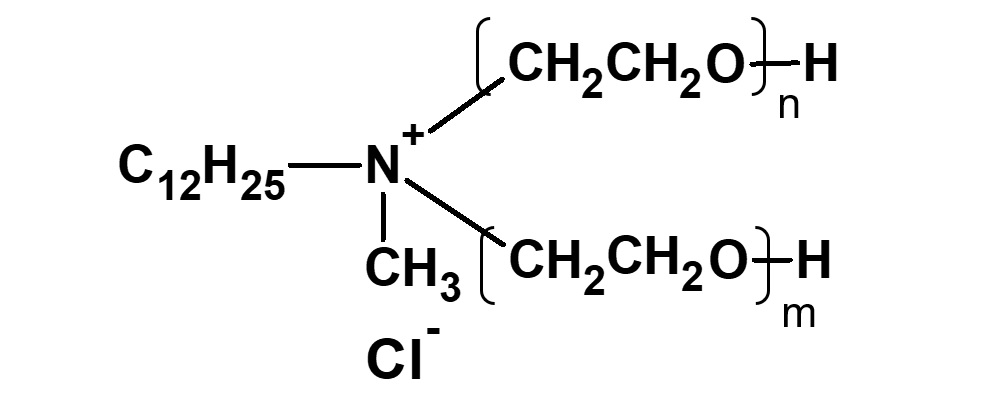
Fig. General structure of amphoteric surfactants
Examples of amphoteric surfactants
Anionic surfactants command a major share of the household detergent market, including soaps, and cationic surfactants are well- known as germicides for disinfection and as finishing agents for synthetic fibers such as nylon. Then, what is a familiar example of an amphoteric surfactant?
It is an important ingredient in cosmetics, especially shampoo. One of the most popular amphoteric surfactants is cocoamidopropyl betaine, derived from coconut fatty acid and dimethyl aminopropylamine.
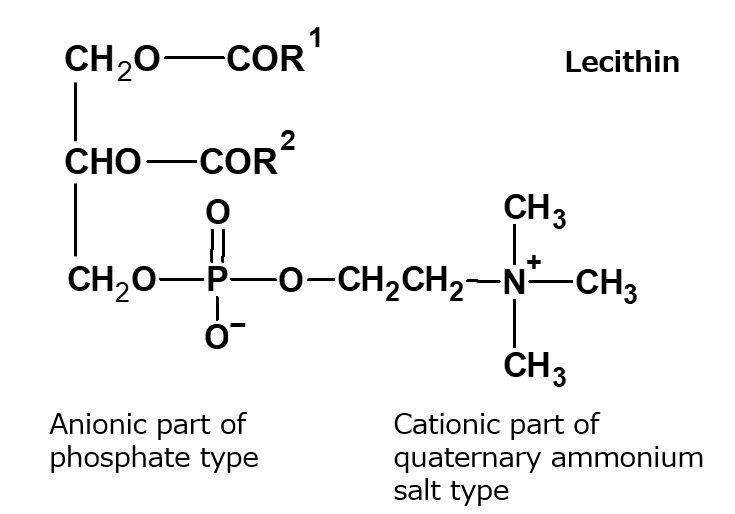
Fig. Structure of Lecithin
Natural amphoteric surfactant: lecithin
The lecithin contained in egg yolk is a natural amphoteric surfactant.
It is indispensable in the preparation of mayonnaise.
Lecithin is an amphoteric surfactant that possesses a phosphate type for its anionic part and a quaternary ammonium salt type for its cationic part As it has two hydrophobic groups in one molecule, it is almost insoluble in water, but it exhibits high interfacial activity in emulsifying oils.
Classification of Amphoteric Surfactants
Although only limited kinds of amphoteric surfactants are used practically, many kinds are conceivable. More concretely, as amphoteric surfactants are composed of anion-cation combinations, the theoretical number of amphoteric surfactants that could be synthesized should be at least as large as the number of kinds of anionics multiplied by that of cationics.
In many practical cases, however, either amine salts or quaternary ammonium salts are used as a hydrophilic group with a cationic property. Therefore, it is convenient to classify an amphoteric surfactant by the type of its anionic part.
The classification of amphoteric surfactants by type of anionic portion is summarized below.
Classification of amphoteric surfactants
| Amphoteric surfactants | Carboxylate type amphoterics | Amino acid type amphoterics Example:R-NHーCH2CH2COOH |
| Betaine type amphoterics Example:  |
||
| Sulfate type amphoterics | ||
| Sulfonate type amphoterics | ||
| Phosphate type amphoterics | ||
However, this table represents experimentally developed amphoteric surfactants only. In practice, most amphoteric surfactants currently on the market are carboxylate type. In this page, therefore, discussion is focused on carboxylate type amphoteric surfactants.
A carboxylate type amphoteric surfactant is one that possesses a carboxyl group as its anionic part. Those possessing an amine salt type cationic part are called amino acid type amphoteric surfactants, and those possessing a quaternary ammonium salt type cationic part are called betaine type amphoteric surfactants (As shown in table above).
Amino acid type amphoteric surfactants
A compound with the following composition is produced when one mole of methyl acrylate (CH2 = CHCOOCH3) is added during stirring to one mole of higher alkylamine (C12 to C18) such as lauryl amine that has been melted by heating to 60 to 70 °C.
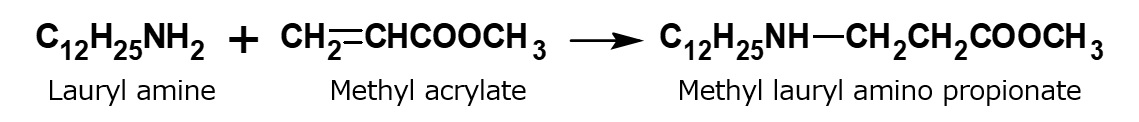
Fig. Synthesis of methyl lauryl amino propionate (secondary amine)
The methyl lauryl amino propionate thus produced is a secondary amine, although it has a slightly complex structure. Therefore, it yields water-soluble cationic surfactants by neutralization with an acid such as hydrochloric acid.
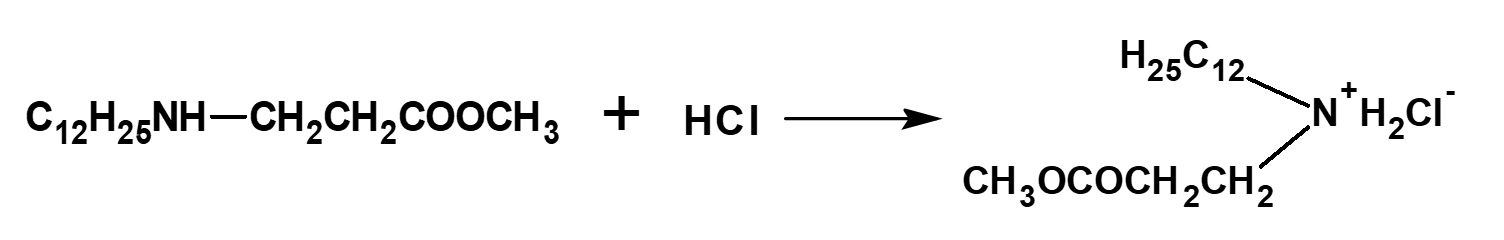
Fig. Neutralization reaction of methyl lauryl amino propionate (cationic surfactant)
However, by focusing on the ester linkage rather than the amine, it becomes apparent that the carboxyl group can be regenerated by saponification with alkali. In this way, an excellent amino acid type surfactant can be obtained.

Fig. Formation reaction of sodium lauryl amino propionate (amino acid type amphoteric surfactant)
The saponification can be carried out easily by adding one mole of aqueous sodium hydroxide solution, slowly, during stirring, to one mole of methyl lauryl amino propionate, in a beaker heated in a boiling water bath. The resultant sodium lauryl amino propionate is easily soluble in water, yielding a transparent solution. Its aqueous solution foams well, and is fairly alkaline because its molecule contains not only sodium carboxylate, as in the case of soap, but also a free secondary amino group.
In an alkaline solution, as shown above, the amino group of an amino acid type amphoteric surfactant performs a relatively minor function as a hydrophilic group because it does not exist as a salt, and the hydrophilic property of the surfactant is attributable mainly to its carboxylate group. Therefore, amphoteric surfactants of this type exhibit properties similar to those of anionic surfactants in alkaline solution.
Behavior of aqueous amino acid-type amphoteric surfactant solutions upon neutralization with hydrochloric acid
Then, what will happen if the alkaline aqueous solution of an amino acid type amphoteric surfactant is gradually neutralized with an acid such as hydrochloric acid ?
When hydrochloric acid is gradually added to the alkaline solution during stirring, nothing happens until the solution is almost neutralized, but precipitation occurs when the solution becomes slightly acid. Regardless, when the solution is fully acidic, and there is excess hydrochloric acid, the precipitation dissolves as if by magic, and a transparent solution is obtained once more.
That is to say, amphoteric surfactants precipitate due to a reduction in hydrophilic property caused by the formation of internal salts, and they dissolve again due to the formation of amine salts with an excess of hydrochloric acid.
In this a manner, amino acid type amphoteric surfactants behave like anionic surfactants under alkaline conditions, and they behave like cationic surfactants under acidic conditions. At the isoelectric point where their anionic and cationic properties are balanced, they precipitate due to the decreased hydrophilic property. The isoelectric point in this case is located on the slightly acidic side.
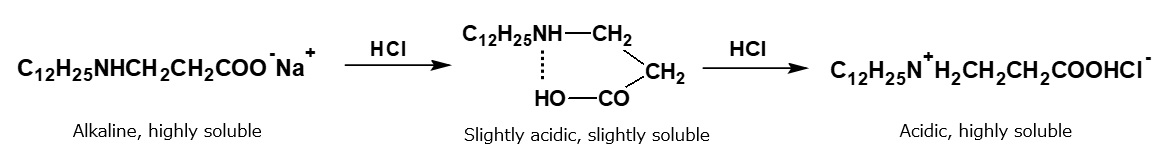
Fig. Behavior of alkaline aqueous amino acid-type amphoteric surfactant solutions upon neutralization with hydrochloric acid
Applications and Synthesis of Sodium Lauryl Amino Propionate and Other Amino Acid Type Amphoteric Surfactants
Sodium lauryl amino propionate has fairly good detergency and is used as a specialty detergent. There are a variety of products in this category with different lengths of alkyl groups and different positions and numbers of amino groups and carboxyl groups. On the whole, they have similar general properties to those shown in the above example, but their applications are diverse.
In addition to the process mentioned above, amphoteric surfactants of alkyl amino propionate type can be synthesized by an alternative process using acrylonitrile, which is more economical.
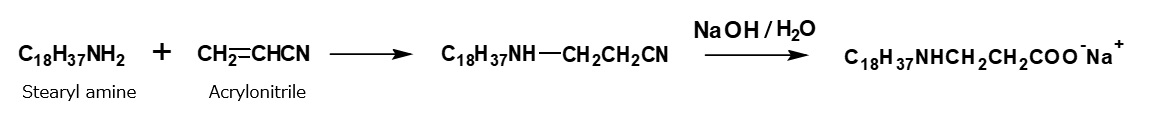
Fig. Synthesis of sodium stearyl propionate (method using acrylnitrile)
Surfactants with a similar structure but with one less methylene group (-CH2-) between the amino and carboxyl groups can be synthesized easily by reacting a higher alkylamine with an aqueous sodium mono chloroacetate solution.
Some amino acid type amphoteric surfactants with this structure are used as germicides with the feature of less toxicity than cationic products.
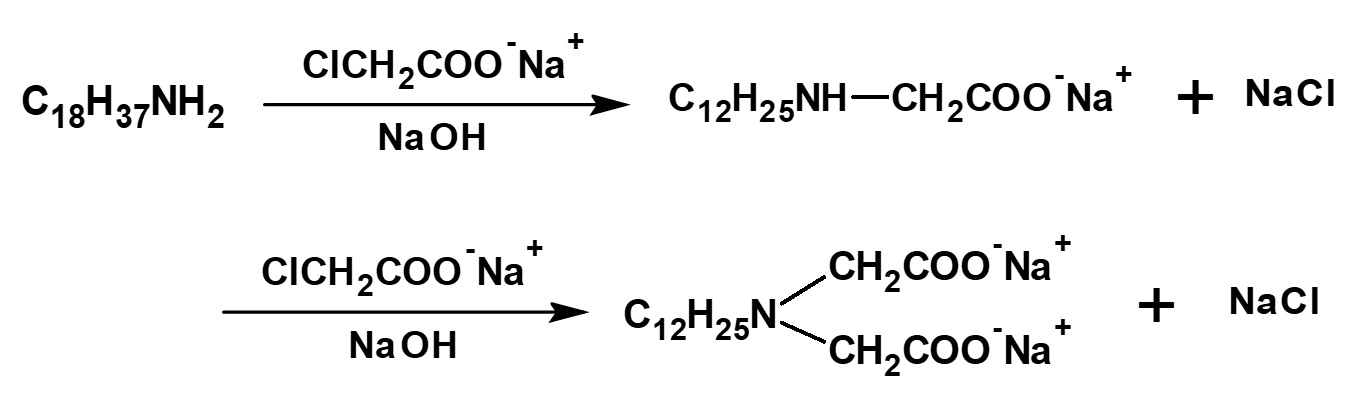
Fig. Reaction of lauryl amine with sodium monochloroacetate
Betaine Type Amphoteric Surfactants
Betaine and betaine surfactants
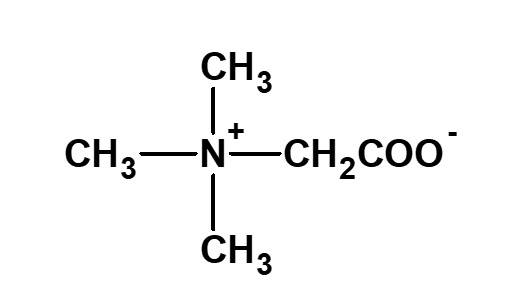
Fig. Structure of betaine
Betaine type amphoteric surfactants are those that have concurrently a quaternary ammonium salt as its cationic part and carboxylate as its anionic part. Betaine is a compound that normally has the structure on the left.
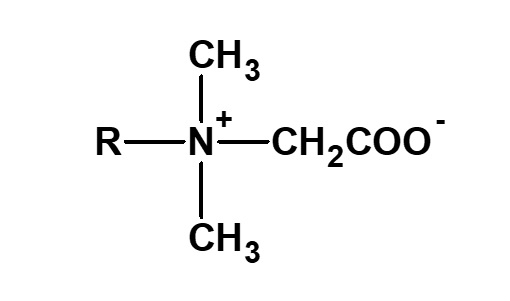
Fig. General structure of betaine
Therefore, betaine type surfactants are generally those compounds with structures similar to betaine, in which one methyl group is replaced by a long chain alkyl group. Of these, the compounds on the left (R: alkyl group of C12 to C18) have the simplest structure while still being of practical use.
For instance, a clear solution of lauryl dimethyl betaine is obtained by mixing aqueous solutions of 1 mole of lauryl dimethyl amine and 1.05 mole of sodium monochloroacetate and having them react during stirring at 60 to 80 °C for a few hours.
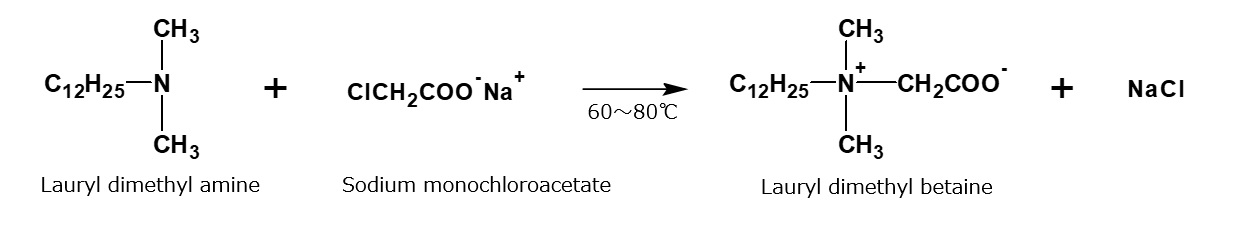
Fig. Formation reaction of lauryl dimethyl betaine
Features of lauryl dimethyl betaine
Lauryl dimethyl betaine dissolves in water transparently and foams well. It has excellent detergency and can be considered a representative betaine type amphoteric surfactant.
The largest difference between betaine type and amino acid type amphoteric surfactants is that betaine type surfactants dissolve in water easily, regardless of whether the betaine type surfactant is acidic, neutral or alkaline in nature. Therefore, in the case of betaine type surfactants, there is almost no danger of precipitation even at the isoelectric point, and there is the added advantage of being usable across the entire pH range.
Dimethyl stearyl betaine, dihydroxyethyl lauryl betaine
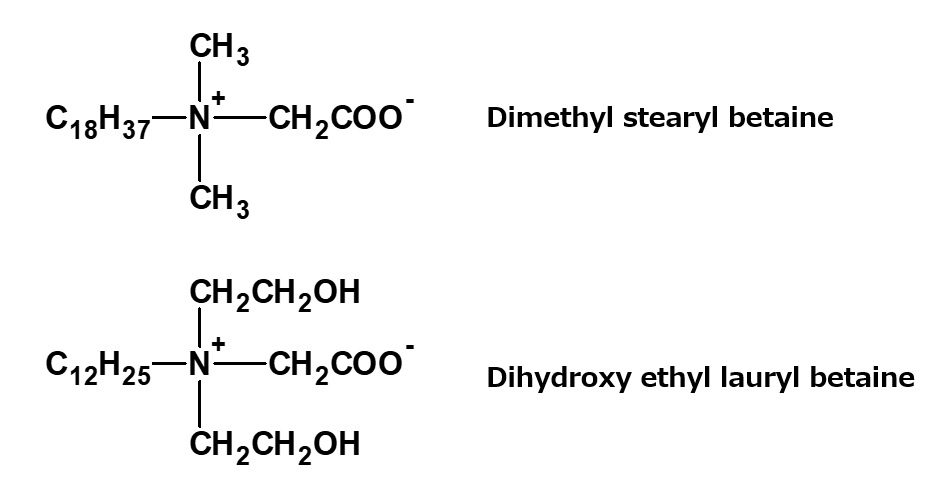
Fig. Dimethyl stearyl betaine, dihydroxyethyl lauryl betaine
Various kinds of betaine type amphoteric surfactants can be synthesized by a process similar to that used for lauryl dimethyl betaine. Important examples of these products are on the left.
Similarly to lauryldimethyl betaine, both of the products on the left are used as dyeing auxiliaries and antistatic agents.
Amphoteric surfactants (cocoacid amidopropyl betaine, lauryl imidazolinium betaine) used as shampoo base
Cocoamidopropyl betaine and laurylimidazolinium betaine are widely used as shampoo bases. These amphoteric surfactants are manufactured from fattyamide amine and alkylimidazoline, respectively, through the reactions indicated in the following reaction formula. However, alkylimidazolinium betaine is actually a complex mixture of products resulting from a cleavage of the imidazoline ring.
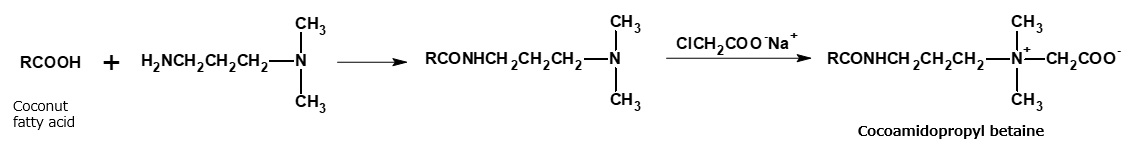
Fig. Synthetic route of coconaut fatty acid amidopropyl betaine
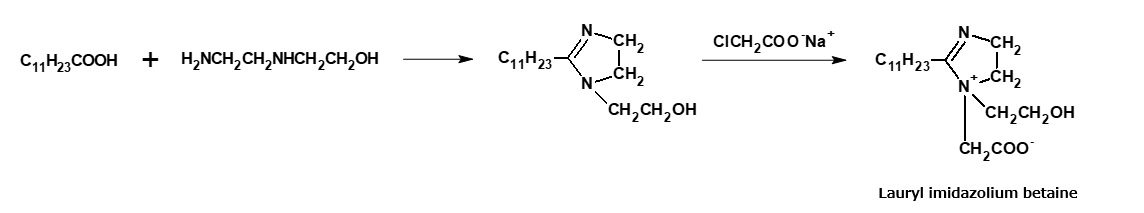
Fig. Synthetic route of lauryl imidazolinium betaine
Summary of Amphoteric Surfactants
A surfactant consisting of an anionic part and a cationic part in its molecule is generally referred to as an amphoteric surfactant. In this class of surfactants, there are various types such as the carboxylate type and the sulfonate type. The most familiar is the carboxylate type, which is subdivided into the amino acid type and the betaine type.
Amino acid type surfactants tend to precipitate at the isoelectric point, but betaine type products dissolve in water relatively easily even at the isoelectric point. In general, the betaine type is better than the amino acid type.
Among these carboxylate-type amphoteric surfactants, the more commonly used forms can be classified based on the raw materials of their hydrophilic and hydrophobic groups, as shown in the table below.
Classification of carboxylate-type amphoteric surfactants
| Hydrophobic raw material | ||||
|---|---|---|---|---|
| Primary amine R-NH2 Secondary amine |
Tertiary amine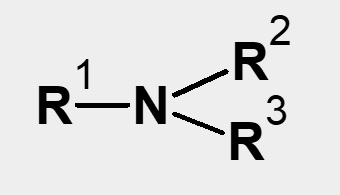 |
Fatty acid RCOOH Higher alkyl chloride RCl |
||
| Hydrophilic raw material |
Sodium monochloroacetate |
Amino acid type | Betaine type | - |
| Methyl acrylate, or acrylonitrile with NaOH |
Amino acid type | - | - | |
| Polyethylenepolyamine and sodium monochloroacetate |
- | - | Amino acid type | |
| Hydroxyethylethylenediamine and sodium monochloroacetate |
- | - | Betaine type | |
Consumption of amphoteric surfactants is small in comparison to other surfactants. However, if you encounter difficulties with regard to surfactant application, it may be more rewarding than expected to consider using amphoteric surfactants.
Related products & topics
Related products

- Cosmetics raw materials
Amino Acid Type Highly Functional Amphoteric Surfactant for Shampoo "PIUSERIA® AMC"
"PIUSERIA® AMC" prevents itching and dandruff by reducing the amount of surfactant residue on the skin during shampooing.

- Antimicrobial agent
Amphoteric surfactant-type antimicrobial agent "LEBON T-2"
By blending with amphoteric surfactants and non-ionic surfactants, a sanitizing detergent with both antimicrobial and cleaning power can be adjusted.

- Nonionic surfactant
Polyoxyalkylene alkylamine-based surfactant "PUREMEEL EP-300S"
Base detergent for clothes with excellent cleaning power against grease and oil stains
| Links to Sanyo Chemical's corprate site |
|---|
Amino Acid Type Highly Functional Amphoteric Surfactant for Shampoo "PIUSERIA® AMC"PIUSERIA AMC: Sodium Lauramino Propionate Amphoteric Surfactants "LEBON"LEBON 2000: Coconut oil fatty acid amidopropyldimethylaminoacetic acid betaine |
Topics





References
T.Fujimonto, Introduction to Surfactants, Sanyo Chemical Industries, ltd.
This page has been prepared solely for information purposes.
Sanyo Chemical Industries, Ltd. extends no warranties and makes no representations as to the accuracy or completeness of the information contained herein, and assumes no responsibility regarding the suitability of this information for any intended purposes or for any consequences of using this information.
Any product information in this brochure is without obligation and commitment, and is subject to change at any time without prior notice.
Consequently anyone acting on information contained in this brochure does so entirely at his/her own risk.In particular, final determination of suitability of any material described in this brochure, including patent liability for intended applications, is the sole responsibility of the user. Such materials may present unknown health hazards and should be used with caution. Although certain hazards may be described in this brochure, Sanyo Chemical Industries, Ltd. cannot guarantee that these are the only hazards that exist.





